
The PICO framework for framing systematic review research questions
May 28, 2021
Publications of Clinical trials in Scientific Journals – Mandatory
June 3, 2021In brief:
The area of medical research is constantly developing, with more data being collected every day. Several studies in clinical research have been classed as descriptive or analytic. Cross-sectional research, ecological correlational studies, case report writing, case series report writing, and surveillance studies are examples of descriptive studies. On the other hand, analytical investigations to investigate a hypothesis concerning a causal link between exposure and result. Because this article focuses on case reports and case series, the analysis will be limited to only those types of research.
Introduction
The clinical case report has a longstanding tradition in the medical literature. While the scientific value of case reports has diminished as more modern research methods have gained traction, they are still published in many medical journals. Some researchers argue that it has minimal relevance for medical advancement, while others say it is underestimated. We hoped to give multiple perspectives on the benefits and drawbacks of the case report genre.
A case report typically consists of one or two cases. A case series or case series report typically includes three to 10 cases. Naturalistic and descriptive case reports are the most common. They can, however, be speculative and experimental at times. Recognizing innovations is arguably the most significant advantage of case reporting. It is the sole means to report unexpected, uncontrolled observations on symptoms, clinical findings, illness progression, complications of therapies, disease linkages, pharmacological side effects, and so on. In summary, anything unusual or previously unseen may be essential for the medical community and should be disclosed. A case report may sensitize readers, making it easier to discover similar or identical instances.

When to consider a Case Series?
If you plan to publish your medical case study report writing and case series, keep in mind that your reports should be the first to document medical breakthroughs, such as reporting rare diseases in recognizing adverse or beneficial outcomes of intervention or novel diagnostic or therapeutic strategies. Furthermore, the major goal was to develop ideas that might be investigated in future research with higher methodological rigour. As a result, case series may be used to evaluate therapy safety and diagnostic accuracy, and no control group is required with appropriate long-term follow-up. There is a requirement for preliminary evidence to build the notion that a therapy may be beneficial to have your research financed. In this scenario, these reports are frequently the first level of evidence.
When is a High Acceptance Rate Possible?
A case series generally incorporates information gathered from multiple sources and includes one or more specifics about the disease, treatment, diagnostic procedures, and so on, whereas a case report has much less data and may consist of detailed demographic information. Acceptance of the case series takes longer than acceptance of the case report by any publisher. Case reports are professional narratives that give feedback on clinical practice standards, a framework for adverse occurrences, and early cost and effectiveness indicators.
Merits and Demerits
Both case series and best-case report writing have advantages and disadvantages. Case series are extremely valuable in rare cases where a new type of sickness or ailment impacts the person. Furthermore, the publishers may make a few concessions while assessing a case series because of the biased content based on the editors’ personal beliefs. Moreover, case series have excellent external validity, little influence on the treatment choice process, a diverse variety of patients, and are affordable. In terms of limitations, the clinical case study report writing lacked comparison groups, and data collection was frequently insufficient, prone to bias, selection bias, and measurement bias. Case reports, on the other hand, enable healthcare practitioners to comprehend the instances and take less time to create. However, case reports may not contain all the pertinent and minute facts required to treat the individuals. A case series does not use randomization, making it more efficient and cost-effective.
Table 1: Role of case reports/series in the medical literature
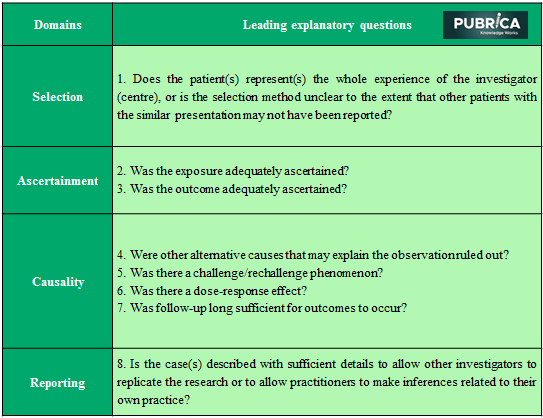
Criteria
A few criteria need to be followed whenever a case series is developed.
- Title – Include the terms “case series” and the area of concentration in the title.
- Abstract – Introduction – Emphasize the case series’ unifying theme.
- Methods – what was done, how and when.
- Results – what was found.
- Conclusion – what we have learned.
- Introduction – emphasize the scientific background and rationale.
- Design –the research registry number (ResearchRegistry.com or ClinicalTrials.gov or ISRCTN) list the intended population, process manner, and end outcome.
(ii) Analysis – While analyzing, descriptive statistics and techniques must be employed, and no comparison tests yielding p values should be performed.
(iii) Reporting – Every follow-up in a case series must provide the topic information and progress, comprehensive details, and analysis.
Table 2: Tool for evaluating the methodological quality of case reports and case series
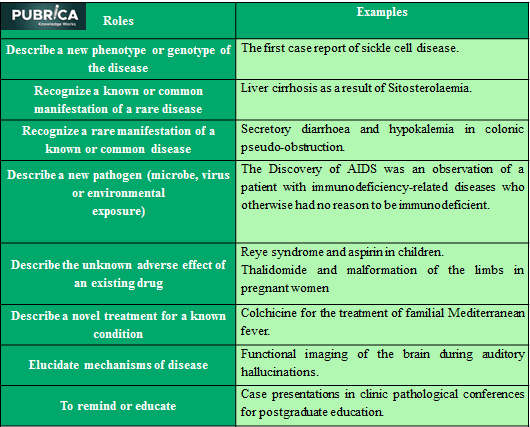
Conclusion
In the clinical research arena, both case series and case reports are intended for use by healthcare practitioners. Aside from the differences between a case series and a case report, they provide reliable information with prompt intervention. If both are adequately prepared from the start, they can even be used as alternatives to another level of studies with greater confidence than the former. Reporting case series in a uniform and statistically suitable manner would allow for accurate interpretation and future meta-analyses to provide long-term outcome estimates.
About Pubrica
Pubrica’s team of researchers and writers create scientific and medical research articles, which may be important resources for authors and practitioners. Pubrica medical writers assist you in creating and revising the introduction by alerting the reader to gaps in the chosen study subject. Our professionals understand the order in which the hypothesis topic is followed by the broad subject, the issue, and the backdrop.
References
- Konala, V. M., Adapa, S., Naramala, S., Chenna, A., Lamichhane, S., Garlapati, P. R., … &Gayam, V. (2020). A case series of patients coinfected with influenza and COVID-19. Journal of investigative medicine high impact case reports, 8, 2324709620934674.
- Tahvildari, A., Arbabi, M., Farsi, Y., Jamshidi, P., Hasanzadeh, S., Calcagno, T. M., … &Mirsaeidi, M. (2020). Clinical features, diagnosis, and treatment of COVID-19 in hospitalized patients: a systematic review of case reports and case series. Frontiers in medicine, 7, 231.
- Nissen, Trygve, and Rolf Wynn. “The clinical case report: a review of its merits and limitations.” BMC research notes 7.1 (2014): 1-7.



