
How many patients does case series should have? In comparison to case reports
June 2, 2021
What data to Extract for systematic review?
June 4, 2021Introduction
The meaning of research is “an endeavour to find realities by study or research.” Undergraduates need to learn research for a solid establishment. Postgraduates need research philosophy for proposal, and clinical instructors are guides for leading their proposition. Professionals need to get research, as they need to manage an assortment of cases. Strategy producers use research for outlining strategies while executives take choices with the assistance of exploration results. So, research information is required for all clinical experts and the fields identified with medication. Research paper Publishing is very important in the academic career as researches provide the researcher with information and knowledge
Clinical Trials is devoted to propelling information on the plan and direction of clinical trials related research techniques. Covering the plan, direct investigation, synthesis and assessment of key approaches, the diary stays on the cusp of the most recent themes, including morals, guideline and policy impact.
Research paper publication of clinical trials
Despite their limits, randomized trials address the benchmark to deal with finding out about the “adequacy” of a specific treatment. Indeed, in the period of proof-based medication, the CT has been enthroned at the most significant level of the highest order of what has been demonstrated.
1. Registration
The ICMJE’s clinical trial registration strategy is definite in the progression of publications. Momentarily, the ICMJE requires and suggests that all clinical diary editors require the enlistment of clinical preliminaries in a public preliminaries vault at or before the hour of first persistent enlistment as a state of thought for distribution. Editors mentioning incorporating their diary on the ICMJE site rundown of distributions that follow ICMJE direction ought to perceive that the posting suggests implementation by the diary of ICMJE’s trials enlistment strategy.
Currently Available Registries
1. The US clinicaltrials.gov registry meets all ICMJE necessities. This information base, created by the National Library of Medicine, is accessible on the web. Even though it relies upon the FDA and the National Institute of Health, it allows the incorporation of global trials. Some European analysts have criticized the vault as being excessively focused on US CTs and not joining data about eventual outcomes.
2. A British privately owned business (Current Controlled Trials) built up the possibility of the standard worldwide library number. In late 2005, responsibility for information base was moved to a non-benefit making association satisfying ICMJE necessities. Presently, this registry (International Standard Randomized Controlled Trial Number) is likewise substantial from an article perspective.
3. The European Community, in a particular harmonization mandate (2001/20/CT) presented enactment that made it required to enrol “clinical examinations about clinical items for human use” and built up the Eudora CT information base constrained by the European Medicines Agency. Albeit this data set could be extremely helpful for European scientists, right now it doesn’t agree with some ICMJE prerequisites as it is a secret register, simply accessible to administrative offices and financing associations.
6. At last, the WHO has built up a worldwide “stage” to arrange CT vaults and accept the administration in this activity. The WHO works together with different associations on projects bound to ensure agreement over the base information contained in the library, the unwavering quality of the data enlisted, and the execution of a solitary global system of numeration.
2. Data Sharing
The ICMJE’s data sharing explanation strategy is nitty-gritty in a publication.
1. As of July 1 2018 compositions submitted to ICMJE journals that report the consequences of clinical trials should contain a data sharing articulation as portrayed beneath.
2. Clinical trials that start selecting members on or after January 1 2019, should incorporate an information-sharing arrangement in the preliminary’s enlistment. The ICMJE’s strategy in regards to preliminary enrollment is clarified previously. On the off chance that the information-sharing arrangement changes after enrollment, this should be reflected in the explanation submitted and distributed with the composition and refreshed in the library record.
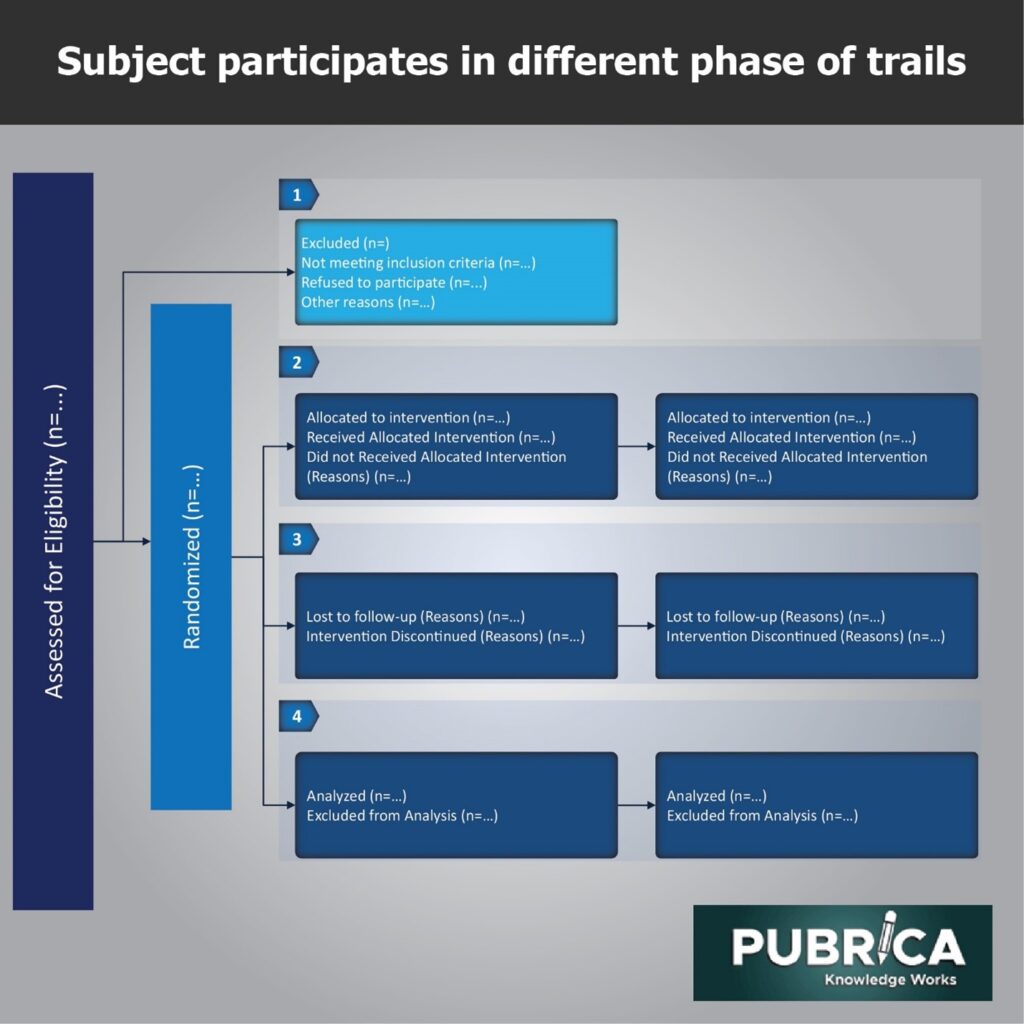
Figure: Flowchart of subjects participates in different phases of trials.
Ethical Considerations
Unfortunately, the public possibly hears about clinical trials when something turns out badly even though many clinical trials are occurring worldwide at any one time with no huge, unfriendly occasions happening. It is because there are numerous actions set up to ensure volunteers and patients as follows.
Institutional Review Boards (IRBs) or Ethics Committees (ECs):
To secure volunteers and patients taking part in clinical preliminaries, the subtleties, everything being equal, should be endorsed by an Independent morals advisory group before any preliminary may begin.
ICF: Before enrolment into the research, members should know all data of clinical research. These formational records called an educate assent structure planned to ensure members and ought to give study-related data (possible dangers, benefits and so forth). The educated assent measure is planned to ensure members. It ought to give sufficient data to an individual to comprehend the dangers of, possible advantages of, and options in contrast to the investigation.
Conclusion
Authors and journal editors cling to conditions set out by the International Committee of Medical Journal Editors. More persevering information sharing is empowered through forthcoming preliminary enlistment and preliminary detailing sites. All in all, clinical researches are intended to add to clinical information identified with the treatment, finding, and anticipation of infections or conditions. Pubrica offers Research and Scientific Journal Publication Support Services by the UK experts at all the way from Journal Selection to Post-submission
References:
- Ross JS, Mocanu M, Lampropulos JF, Tse T, Krumholz HM. Time to publication among completed clinical trials. JAMA Intern Med; 2013 [cited 2017 May 19];173(9):825.
- Scherer RW, Langenberg P, von Elm E. Full publication of results initially presented in abstracts. In: Scherer RW, editor. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley & Sons, Ltd; 2007 [cited 2017 May 19].
- De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med [Internet]. Massachusetts Medical Society; 2004 [cited 2017 May 19];351(12):1250–1251.
- World Medical Association (WMA). Declaration of Helsinki. Ethical principles for medical research involving human subjects. JahrbfürWiss und Ethik [Internet]. 2009 [cited 2017 May 19];14(1).
- National Institutes of Health. NIH policy on dissemination of NIH-funded clinical trial information. Fed Regist. 2016;81:64922–8.
- Department of Health and Human Services. Final rule—clinical trials registration and results information submission. Fed Regist. 2016;81:64981–5157.
- Scott A, Rucklidge JJ, Mulder RT, Strech D, Mann H, Berlin J. Is mandatory prospective trial registration working to prevent publication of unregistered trials and selective outcome reporting? An observational study of five psychiatry journals that mandate prospective clinical trial registration. Wicherts JM, editor. PLoS One [Internet]. Public Library of Science; 2015 [cited 2017 May 19];10(8):e0133718




What are the key points to reflect while considering the case report series in BMJ?
Read more