Publications of Clinical trials in Scientific Journals – Mandatory
June 3, 2021
How to overcome regulatory and ethical challenges regarding medical device?
June 8, 2021In-Brief:
Systematic literature (Qualitative, non-meta-analysis) review writing is a protocol-driven process that demands researchers to extract, analyse and present an exhaustive summary of the latest yet apt literature for their specific studies in the prescribed format along with bias/evidence quality figures. It primarily focuses on clear, structured questions that need to be answered using an in-depth search strategy.
Systematic review authors should decide ahead of time what information will be needed for their precise review and build up a technique for acquiring them. Although there are several software’s (e.g., Covidence, Colandr, Rayyan, CREBP, EPPI-Reviewer 4, Distiller, JBI SUMARI tool, Systematic Review Data Repository (SRDR), Systematic Review Toolbox available for collecting data, its researchers’ knowledge and skills play a major role in the extraction.
What should Extracted data should help us to drive?
The data extracted should sufficiently depict the included investigations, support the development of tables and figures, encourage the risk of bias assessment, and empower syntheses and meta-analyses. Review authors ought to acquaint themselves with detailing rules for systematic review and the PRISMA statement; to guarantee that significant components and areas are incorporated.
Items to be considered for data extraction and writing a systematic review
Data extractor name, data extraction date, identification features of a report from which we are going to extract the data is also part of the extraction process.
- Eligibility and Documenting decisions
- Confirm qualification of the examination for the review including reference (first author/year/journal citation)
- Visually scanning references lists from relevant studies
- Hand searching key journals from identified studies
- Contacting study authors, experts, manufacturers, and other organizations
- Citation searching
- Reasons for exclusion of the study.
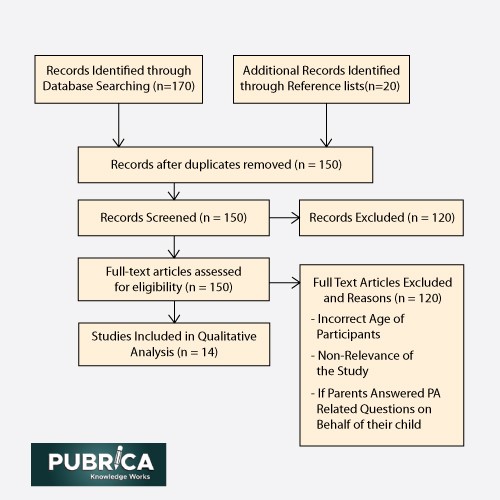
Figure: PRISMA flow chart depicting the article filtering process.4
2. Study Location and demographic details
- Country
- Location
- Race / Religion if its important
- Gender
- Age
- Medical Condition (DM or HTN)
3. Study techniques
Study plan:
- Parallel, factorial, hybrid, bunch parts of the plan for randomized preliminaries, and additionally study configuration highlights for non-randomized examinations.
- Single or multicenter study; if multicenter, a number of enlisting focuses.
- Recruitment and testing methods utilized (counting at the degree of individual members and bunches/destinations if significant).
- Enrolment start and end dates; length of member follow-up.
- Details of irregular arrangement age, distribution grouping covering, and veiling for randomized preliminaries, and strategies used to forestall and control for perplexing, choice predispositions, and data inclinations for non-randomized investigations.
- Methods used to forestall and address missing information.
4. Statistical analysis:
- Analysis unit (for example, singular member, Centre, town, body part)
- Statistical techniques utilized whenever registered impact gauges are separated from reports, incorporating any covariates remembered for the measurable model
- Likelihood of revealing and other biases.
- Funding sources or other material help for the study.
- Authors’ monetary relationship and other possible irreconcilable circumstances.
5. Participants
- Setting
- Region(s) and country/nations from which study members were enlisted
- Study qualification measures, including symptomatic rules
- Qualities of members toward the start (or gauge) of the investigation (for example, age, sex, comorbidity, financial status).
6. Intervention
Depiction of the intervention(s) and examination intervention(s), preferably with adequate detail for replication:
- Components, courses of conveyance, portions, timing, recurrence, intercession conventions, length of mediation
- Factors pertinent to execution (for example, staff capabilities, hardware prerequisites)
- The integrity of intercessions (for example, how much indicated methodology or segments of the mediation were executed as arranged)
- Description of co-intercessions
- Definition of ‘control’ gatherings (for example, no intercession, fake treatment, negligibly dynamic comparator, or segments of regular consideration)
- Components, portion, timing, recurrence
- For observational examinations: depiction of how intercession status was evaluated; length of openness, aggregate openness.
7. Outcomes
For each pre-indicated result area (for example, uneasiness) in the systematic review:
- Whether there is proof that the resulting space was evaluated (particularly significant if the result was surveyed yet the outcomes not introduced.
- Measurement apparatus or instrument (counting meaning of clinical results or endpoints); for a scale, name of the scale (for example, the Hamilton Anxiety Rating Scale), upper and lower cutoff points, and whether a high or low score is good, meanings of any limits if fitting.
- Specific metric (for example, post-intercession anxiety, or change in uneasiness from pattern to a post-meditation time point, or post-meditation presence of nervousness (yes/no))
- Method of total (for example, the mean and standard deviation of tension scores in each gathering or extent of individuals with nervousness)
- Timing of result estimations (for example, appraisals at the end of the eight-week intercession period, occasions happening during the eight-week mediation period)
- Adverse results need exceptional consideration relying upon whether they are gathered methodically or non-deliberately (for example, by deliberate report).
8. Results
- For each group, and for every result at each time point: number of members arbitrarily relegated and remembered for the study; and number of members who pulled out, were lost to follow-up or were rejected (with purposes behind each)
- Summary information for each group (for example, 2×2 table for dichotomous information; means and standard deviations for consistent information)
- Between-bunch appraises that evaluate the impact of the intercession on the result, and their accuracy (for example, hazard proportion, chances proportion, and mean contrast)
- If subgroup investigation is arranged, similar data should be extricated for every member subgroup.
9. Miscellaneous
- Key conclusion of the author.
- Reference to other pertinent investigations
- Correspondence required
- Miscellaneous remarks from the author of the study or by the review authors.
Other information to collect
The authors gather the critical finishes of the included study as detailed by its authors. It isn’t important to report these ends in the survey, yet they ought to be utilized to confirm the consequences of the study attempted by the review authors, especially corresponding to the course of impact. Further remarks by the study authors, for instance, any clarifications they accommodate startling discoveries, might be noted. References to different studies that are referred to in the investigation report might be helpful, in spite of the fact that review authors ought to know about the chance of reference inclination. Documentation of any correspondence with the examination creators is significant for review straightforwardness.
Conclusion
Preferably, data just should be extricated once and ought to be put away in a safe and stable area for future updates of the survey, whether or not the first review authors or an alternate gathering of authors update the Systematic review. Normalizing and sharing information assortment apparatuses just as information the board frameworks among review authors are working in comparative subject regions can smooth out deliberate review creation. Review authors have the chance to work with trial lists, diary editors, funders, controllers, and different partners to make study information (for example, CSRs, IPD, and some other type of study information) freely accessible, expanding the straightforwardness of study. Pubrica Systematic Review Support Service is a pilot program to support researchers in performing high-quality systematic reviews.
General information
Researcher performing data extraction
Date of data extraction
Identification features of the study:
Record number (to uniquely identify study)
Author
Article title
Citation
Type of publication (e.g. journal article, conference abstract)
Country of origin
Source of funding
Study characteristics
Aim/objectives of the study
Study design
Study inclusion and exclusion criteria
Recruitment procedures used (e.g. details of randomisation, blinding)
Unit of allocation (e.g. participant, GP practice etc.)
Participant characteristics
Characteristics of participants at the beginning of the study e.g.
Age
Gender
Ethnicity
Socio-economic status
Disease characteristics
Co-morbidities
Number of participants in each characteristic category for intervention and control group(s) or mean/median characteristic values (record whether it is the number eligible, enrolled, or randomised that is reported in the study)
Intervention and setting
Setting in which the intervention is delivered
Description of the intervention(s) and control(s) (e.g. dose, route of administration, number of cycles, duration of cycle, care provider, how the intervention was developed, theoretical basis (where relevant))
Description of co-interventions
Outcome data/results
Unit of assessment/analysis
Statistical techniques used
For each pre-specified outcome:
Whether reported
Definition used in study
Measurement tool or method used
Unit of measurement (if appropriate)
Length of follow-up, number and/or times of follow-up measurements
For all intervention group(s) and control group(s):
Number of participants enrolled
Number of participants included in analysis
Number of withdrawals, exclusions, lost to follow-up
Summary outcome data e.g.
Dichotomous: number of events, number of participants
Continuous: mean and standard deviation
Type of analysis used in study (e.g. intention to treat, per protocol)
Results of study analysis e.g.
Dichotomous: odds ratio, risk ratio and confidence intervals, p-value
Continuous: mean difference, confidence intervals
If subgroup analysis is planned the above information on outcome data or results will need to be extracted for each patient subgroup
Additional outcomes
Record details of any additional relevant outcomes reported
Costs
Resource use
Adverse events
NB: Notes fields can be useful for occasional pieces of additional information or important comments that do not easily fit into the format of other fields.
Reference:
- Davis AL, Miller JD. The European Medicines Agency and publication of clinical study report a challenge for the US FDA. JAMA 2017; 317: 905-906.
- Denniston AK, Holland GN, Kidess A, Nussenblatt RB, Okada AA, Rosenbaum JT, Dick AD. Heterogeneity of primary outcome measures used in clinical trials of treatments for intermediate, posterior, and panuveitis. Orphanet Journal of Rare Diseases 2015; 10: 97.
- Lewin S, Hendry M, Chandler J, Oxman AD, Michie S, Shepperd S, Reeves BC, Tugwell P, Hannes K, Rehfuess EA, Welch V, McKenzie JE, Burford B, Petkovic J, Anderson LM, Harris J, Noyes J. Assessing the complexity of interventions within systematic reviews: development, content, and use of a new tool (iCAT_SR). BMC Medical Research Methodology 2017; 17: 76.
- Li G, Abbade LPF, Nwosu I, Jin Y, Leenus A, Maaz M, Wang M, Bhatt M, Zielinski L, Sanger N, Bantoto B, Luo C, Shams I, Shahid H, Chang Y, Sun G, Mbuagbaw L, Samaan Z, Levine MAH, Adachi JD, Thabane L. A scoping review of comparisons between abstracts and full reports in primary biomedical research. BMC Medical Research Methodology 2017; 17: 181.
- Liu ZM, Saldanha IJ, Margolis D, Dumville JC, Cullum NA. Outcomes in Cochrane systematic reviews related to wound care: an investigation into prespecification. Wound Repair and Regeneration 2017; 25: 292-308.