What should you know before you write a Biosimilar Grant Proposal Writing
May 30, 2022
The use of network meta-analysis in the field of physical exercise and health promotion
June 14, 2022In brief
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) report was published in 2009 to aid Systematic Review Writing in publicly reporting why the review was undertaken, what the authors did, and what they discovered. Over the previous decade, advances in systematic review methodologies and language have necessitated an update to the guideline. The PRISMA 2020 statement supersedes the PRISMA 2009 statement and includes updated reporting requirements that take into account changes in research methodology for identifying, selecting, assessing, and synthesizing. The structure and demonstration of the items have been altered to make implementation easier. The PRISMA 2020 abstract-item checklist, which contains reporting suggestions for each item and the PRISMA 2020 abstract checklist and redesigned flow diagrams for original and modified reviews, are all represented in this blog.
Introduction
Conducting a Systematic Review provide several important responsibilities. They can provide summaries of the state of knowledge in a field, from which future research priorities can be identified; they can answer questions that individual studies would otherwise be unable to answer; they can locate imperfections in primary research that should be addressed in future studies, and they can generate or evaluate theories about how or why phenomena occur. As a result, systematic reviews create varied types of knowledge for various review consumers (such as patients, healthcare providers, researchers, and policymakers). To ensure that a systematic review is useful to users, writers should provide a clear, thorough, and accurate description of why the review was conducted, what they did (such as how studies were located and selected), and what they discovered (such as characteristics of contributing studies and results of meta-analyses). Authors can succeed with the help of up-to-date reporting guidelines.
Development of PRISMA 2020
We circulated an initial draft and five versions of the checklist and explanation and elaboration document to co-authors for input during 2019 and 2020. We contacted 22 Systematic Review Writing Services who had indicated an interest in providing comments on the PRISMA 2020 checklist to give their thoughts on the form and terminology used in an early version of the checklist (through an online survey) in April 2020. 15 people provided feedback, which the initial author examined, and any required adjustments were made before the final version was authorized and endorsed by all co-authors.
Scope of the guideline
The PRISMA 2020 declaration was created with systematic reviews of studies that evaluate the impact of health treatments in mind, regardless of the study type. The checklist items, on the other hand, may be included in reports of Clinical Trial Systematic Review Services assessing various treatments (such as social or educational interventions), and many of them can be used in systematic reviews with goals other than evaluating interventions (such as evaluating aetiology, prevalence, or prognosis). PRISMA 2020 is designed for use in systematic reviews that contain or do not include synthesis (e.g., paired meta analysis or other statistical synthesis approaches) (for example, because only one eligible study is identified).
Table 1 PRISMA 2020 for Abstracts checklist
| Section and topic | Item # | Checklist item |
| Title Title | 1 | Identify the report as a systematic review |
| Background Objectives | 2 | Provide an explicit explanation of the review’s principal objective(s) or question(s). |
| Methods Eligibility criteria Information sources Risk of bias Synthesis of results | 3 4 5 6 | Specify the review’s inclusion and exclusion criteria. Specify the information sources (e.g., databases, registrations) that were utilized to find research and the last time each was searched. Specify the procedures used to assess the risk of bias in the included studies. Specify the methods used to present and synthesize results. |
| Results Included studies Synthesis of results | 7 8 | Give the total number of included educations and participants and summarise relevant characteristics of studies. Present the key outcomes’ results, preferably with the number of studies and participants. Report the summary estimate and confidence/credible interval if a meta-analysis was performed. If you’re comparing groups, note the effect’s direction (i.e. which group is favoured). |
| Discussion Limitations of evidence Interpretation | 9 10 | Provide a precise summary of the evidence’s limitations in the evaluation (e.g. study risk of bias, inconsistency and imprecision). Provide a general understanding of the results and important implications. |
| Other Funding Registration | 11 12 | Specify the primary source of funding for the review. Provide the registered name and registration number. |
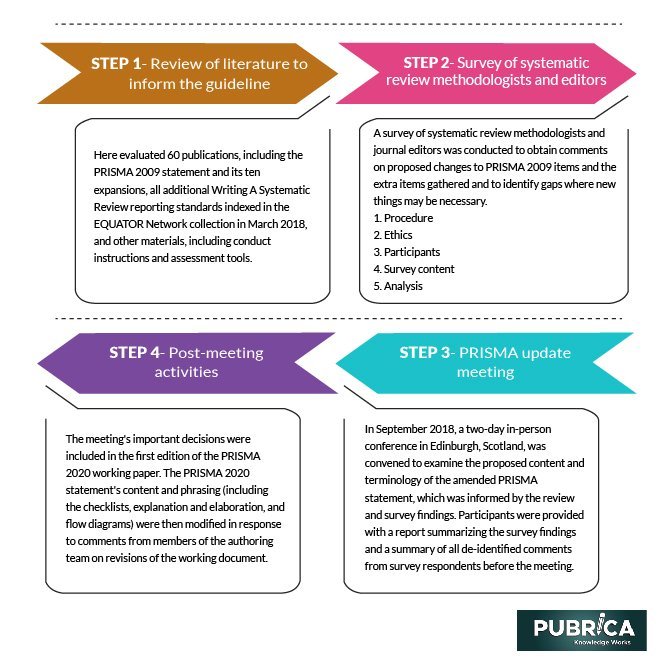
Step 1. Review of literature to inform the guideline
Here evaluated 60 publications, including the PRISMA 2009 statement and its ten expansions, all additional Writing A Systematic Review reporting standards indexed in the EQUATOR Network collection in March 2018, and other materials, including conduct instructions and assessment tools.
Step 2. Survey of systematic review methodologists and editors
A survey of systematic review methodologists and journal editors was conducted to obtain comments on proposed changes to PRISMA 2009 items and the extra items gathered and to identify gaps where new things may be necessary.
- Procedure
- Ethics
- Participants
- Survey content
- Analysis
Step 3. PRISMA update meeting
In September 2018, a two-day in-person conference in Edinburgh, Scotland, was convened to examine the proposed content and terminology of the amended PRISMA statement, which was informed by the review and survey findings. Participants were provided with a report summarizing the survey findings and a summary of all de-identified comments from survey respondents before the meeting.
Step 4. Post-meeting activities
The meeting’s important decisions were included in the first edition of the PRISMA 2020 working paper. The PRISMA 2020 statement’s content and phrasing (including the checklists, explanation and elaboration, and flow diagrams) were then modified in response to comments from members of the authoring team on revisions of the working document.
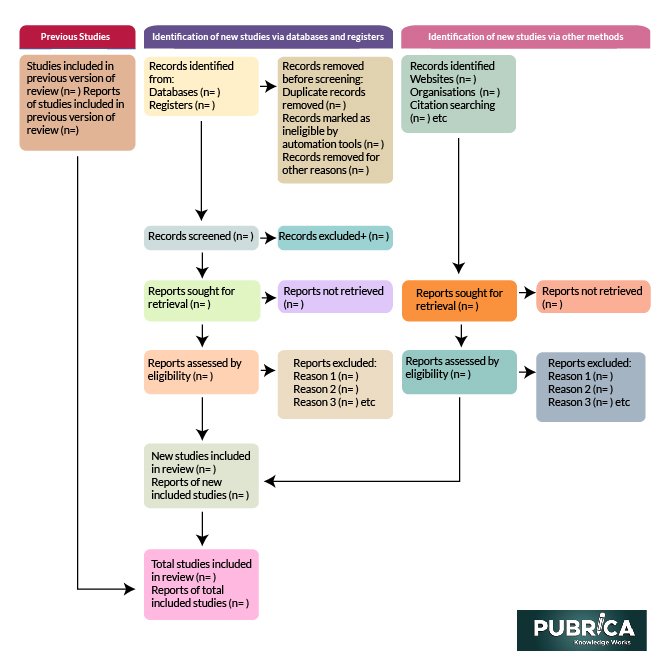
Fig. 1. PRISMA 2020 flow diagram template for systematic reviews
Differences between the PRISMA 2009 and 2020 statement
In some areas, the PRISMA 2020 statement varies from the PRISMA 2009 statement (a list of changes to each item with rationale is provided and the alignment of the checklist items between statements is presented). All checklist items have had their wording changed to accommodate reporting guidance for new and updated methods, to improve author clarity, to facilitate replicability of reviews, to facilitate assessments of the validity and applicability of systematic review specialists, to ensure the item applies to a larger population of studies, to remove redundancy across items, or for any other reason.
Conclusion
The implementation of PRISMA 2020 can benefit a wide range of stakeholders. Complete reporting helps readers assess the methodology’s appropriateness and, as a result, the findings’ credibility. The capacity to evaluate the application of the findings to their situation is made possible by presenting and summarizing aspects of research contributing to a synthesis. Policymakers, managers, and other decision-makers should be able to construct suitable recommendations for practice or policy by describing the certainty of a result in the body of evidence and the consequences of the findings.
About Pubrica
Pubrica’s team of researchers and authors creates scientific and medical research articles that practitioners and authors may use as a valuable resource. By presenting the reader to the deficiencies or gaps in the specified study field, Pubrica medical writers may assist you in writing and editing the introduction. Our professionals are familiar with the framework that follows the broad topic, problem, and background before moving on to a more specific topic to present the hypothesis.
References
- Page, Matthew J., et al. “Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement.” Journal of clinical epidemiology 134 (2021): 103-112.
2.Page, Matthew J., et al. “The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.” International Journal of Surgery 88 (2021): 105906.