FDA classify Medical Devices and how to report device problems: A Systematic Review
April 22, 2022
How to Choose PubMed Journals to Publish Generic Drug Papers?
May 4, 2022In brief
The content and quality determine the value of medical device registries (MDRs); MDRs must have a strong and suitable structure to achieve their goals. MDRs, on the other hand, have no design or content restrictions. This study aims to examine several MDRs in implants and provide best practice suggestions for quality standards in the design and development of these devices by Niederlaender and Christine Kriza (2017), Interdisciplinary C Interdisciplinary Centre for Health Technology Assessment (HTA) and Public Health (IZPH), Friedrich-Alexander-University Erlangen-Nürnberg (FAU). The following recommendations were based on extracted MDRs: MDRs should provide a basic data set and report on the geographic region, data collecting, patient enrollment numbers, registry staff, and data security and confidentiality in Systematic Review Writing.
Introduction
Registries serve an important role in many aspects of the healthcare system. They’re known as prospective observational studies since they collect ongoing and supportive data on well-defined outcomes of attention for analysis and reporting on well-defined effects of interest. Disease-specific registries, country-specific registries, product-specific (branded) registries, and community-based / regional or global registries are all examples of patient registries. “[Registries] can be used to learn about natural history, assess or monitor real-world safety and effectiveness, evaluate care quality and provider performance, and determine cost-effectiveness.”
A systematic literature search performed in databases (Medline, Cochrane Library, Scopus, Embase, CRD York), selected journals and websites identified articles describing either a general MDR structure or the development process of specific registries.
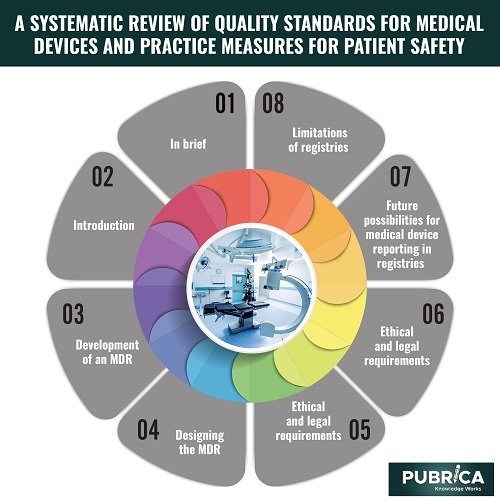
Development of an MDR
All of the registries in this overview defined and outlined their objectives, such as collecting valid data on implant use and related procedures to assess long-term device safety and efficacy, screening for complications and adverse events, measuring failure rates, developing quality improvement activities, evaluating the patient Outcome and identifying patients at risk, and gathering demographic information in Systematic Review Writing Services.
Designing the MDR:
The geographic area covered, the time frame for data collection, the use of national approved codes – as the Scandinavian registries demonstrate –, the routine link to other databases, the composition of the registry team, and the time frame for follow-up are all critical factors in the registry design process. A clinical or professional review would also benefit from conducting a systematic review. Some registries capture data at the point of care, whereas others are only virtual. Data can be gathered through paper-based questionnaires, the Internet, and barcode readers. To enable long-term longitudinal follow-up, registries must interface with electronic health records (EHRs).
Ethical and legal requirements:
Patients are frequently required to provide formal authorization for the registry to utilize their information. The Federal Policy for the Protection of Human Subjects outlines the requirements for obtaining informed consent. Medical professional secrecy and confidentiality protect the patient’s privacy. The essential components of data security are confidentiality, integrity, and availability. When registry data is pseudonymized, and therefore a connection from patient to personal data is feasible, informed consent from patients is usually required to ensure that the register may transmit information back to patients.
Future possibilities for medical device reporting in registries
The scope of registers is evolving, as evidenced by the research reviewed. Home Monitoring transmits to physicians daily systematically, such as by email. The observed events are categorized (medical, system status-related, configuration monitoring), the rates of occurrences are given, and the follow-up visits to the patient. The communicator delivers information from the implanted CRT-D device (cardiac resynchronization treatment) to the home monitoring equipment in the same way. A complete integrated medical device interface system is available for outpatient electronic medical records. However, this reducing method of documenting is only viable if the equipment is electronic. This type of home monitoring cannot be used in stents or hip implants.
Limitations of registries
Although registries in clinical trial systematic review services have a high level of external validity, they can be prone to various biases and systematic mistakes, which must be considered when developing an MDR. There is a possibility of selection bias, information bias (validity of detected data), channelling bias (drugs with comparable therapeutic indications are administered to groups of patients with prognostic differences, including only previously benefitted users of a drug/device), and loss to follow-up. Other biases, such as wandering risk comparisons, confounding by disease severity, depletion of the susceptible, and the immortal time bias, are summarised in his article. As a result, registries must analyze the possibility and severity of deception.
Conclusion
Well-structured registries are an important part of the medical device regulation process and are useful for decision-makers and management. Improvements in the comparability of various registers are also achievable in this manner. In this systematic review, however, only a few studies that outline explicit requirements for the design of an MDR were found. The MDRs’ outcomes can be significantly improved if detailed suggestions are established. These ideas for MDR development criteria will serve as a foundation for future efforts to build standards for medical device registries. This is a step forward in improving surveillance and expanding the use of well-designed registries as early warning systems for identifying and informing people at risk of infection.
About Pubrica
Researchers and authors at Pubrica produce scientific and medical research papers that practitioners and authors may reference. Pubrica medical writers aid you in creating and rewriting the introduction by highlighting the flaws or gaps in the chosen research subject. Our experts understand the framework that leads a broad topic, problem, and background to a small topic where the hypothesis is given.
References
- Niederlaender, Charlotte Susanne, Christine Kriza, and Peter Kolominsky-Rabas. “Quality criteria for medical device registries: best-practice approaches for improving patient safety–a systematic review of international experiences.” Expert Review of Medical Devices 14.1 (2017): 49-64.
- Labek G, Todorov S, Iovanescu L, et al. Outcome after total ankle arthroplasty-results and findings from worldwide arthroplasty registers. Int Orthop. 2013;37:1677-82. Epub 2013/07/09.