
How to write a case report
July 19, 2021
How to Formulate a Researchable Question based on PICOS
July 26, 2021Biomaterials and medical instruments are widely researched and incorporated, which greatly increase the quality of human life, thanks to the rapid advancement of biomedical science and practice. The market for biomaterials and medical devices has risen dramatically as the world’s population ages.With the introduction of novel implant materials, including drug-carrying stents for regenerative medicine, joint repair materials, prostheses, and embedded detection sensors, the global biomaterial market is expanding exponentially. It is probable to advance at a CAGR of 13.7 percent over 2021, reaching a net worth of $130 billion [1].This article discusses biomedical advances and innovations that help researchers to find the research gaps.
1. Emergence of Biomaterials
Polymers are used in facial prostheses, tracheal tubing, kidney and liver sections, heart components, and other biomedical instruments. The ultrahigh molecular weight polyethene (UHMWPE) is used in the knee, hip, and shoulder joints. The polymers used in healthcare are mentioned in Table 1.
Table 1.Polymers commonly used in biomedical applications
| S. no. | Polymer | Application |
| 1 | Cells/polytetrafluoroethylene, Cells/ polyethylene terephthalate, polyethylene terephthalate /collagen | Vascular grafts |
| 2 | Bioglass/ polyurethane , Bioglass/ polysulfones, carbon fibers / polysulfones | Spine cages, plates, rods, screws, discs |
| 3 | Silicone rubber | Finger Joints |
| 4 | Ultrahigh-molecular-weight polyethylene | Knee, Hip, Shoulder Joints, Dental bridges |
| 5 | Polyethylene Terephthalate /Polyurethane, Polyethylene terephthalate /collagen | Abdominal wall prosthesis |
| 6 | Polydimethylsiloxane, polyurethane, polyvinyl chloride | Facial Prostheses |
| 7 | Polymethylmethacrylate | Bone Cement |
Various artificial implants to replace damaged tissues have been created in recent decades, and various implantable biosensors have been used to:
1. Maintain functional physiology by monitoring the human body, including weakened and malfunctioning tissues that artificial substitutes can replace, such as vitreous bodies and joints,[2]
2. Orthopedic implants that facilitate osseointegration and fracture healing[3]
3.Pacemakers are electronic devices that help to regulate irregular heart rhythms[4], stents used to treat arterial stenosis [5]
4. Nerve probes are used to treat and control the electroencephalogram of patients with brain disorders [6] and
5. Patients with chronic diabetes may use continuous blood glucose monitors to track their blood glucose levels in real-time [7].
The concept of a biomaterial’s intrinsic essence has evolved significantly over time, a process that is still in progress (Figure. 1).
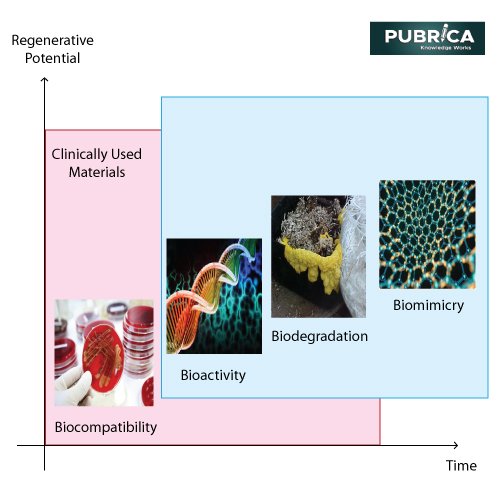
Figure. 1. Evolution of biomaterials [8]
2. Antimicrobial strategy external clinical translation: main collaborators and players
Rapid biomaterial production is fraught with risk. Most sensors and implants are recognised as “alien substances” by the host. The immune system activates dynamic signal cascades during wound-healing procedures, resulting in fibrosis collagen encapsulation on the implanted materials and instruments followed by complications. This process is known as the foreign-body response or foreign-body reaction (FBR), the host body’s natural protective mechanism. Still, it largely affects the function of implanted materials. The foreign-body response, also known as the foreign-body reaction (FBR), is the host body’s normal defence mechanism.Yet, it has a vastoutcome on how embedded materials work.The core collaborators and players in the backend translation of new, enhanced antimicrobial techniques treating biomaterial-associated infections (BAI) through a wide range of applications and patient conditions collaborate in a dynamic relationship that periodically results in new, licenced drugs.The so-called “family of Ps,” which includes patentors (academic and business researchers), manufacturers, payers, patients and clinicians, healthcare suppliers, and policymakers, collaborate with regulatory authorities and legal bodies to decide the emerging innovations that arise and remain clinically adopted.In this respect, these bodies’ interactive functions in restricting and generating medical device advances are summarised in Fig. 6.
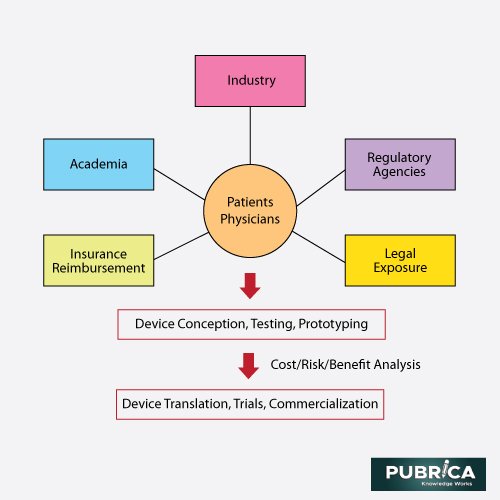
Fig. 6.Antimicrobial methods for biomaterial implants: key collaborators and players in the creation and downstream translation [9]
3. Issues with the partners and players
In a perfect future, biomaterial implant and system inventions, as well as breakthrough improvement techniques, will be patented before publishing, allowing industry incentives to convert these ideas into goods with appropriate rights, exclusivities, and benefit motives.Preclinical and clinical results from novel commercial devices are first submitted to regulatory authorities for clear, direct advice, allowing marketing and patient use clearance.However, 21st-century practises tending to be new since they are subject to significant and distinct stresses from various outlets, many of which complicate accurate, dependable, and timely technological advancement for the benefit of patients. One of the factors influencing is:
– There is a lot of demand to print academic findings quickly because there aren’t many incentives to patent until they’re published. However, for BAI victims, inventions released before successful patent protection are useless.Without certain intellectual property rights and related necessary assurances for return-on-investment to push this translational phase, the industry would be reluctant to gamble new strategies into growth and future production.
4.Future research on biomaterials
The interesting developments on the horizon for biomaterials are listed below:
Immunomodulation is the process of adjusting the immune response to a certain degree. Type 1 diabetes is an infectious disease in which the body’s immune system attacks the pancreas’ insulin-producing cells.Immunomodulating biomaterials could help cure this illness.Researchers recently created an injectable synthetic biomaterial that reversed type 1 diabetes in non-obese diabetic mice, paving the way to create better a biodegradable platform to monitor the disease’s impact.
Injectable biomaterials produce biomedical agents such as medicine, genetic materials, and proteins in greater numbers. They allow for targeted transmission while preventing immune system absorption, allowing for the treatment of various conditions. Injectable biomaterials from engineered and naturally derived materials are being studied to treat bone defects, tumours, and heart attacks.
Orthopaedic implant technology has come a long way in only a few decades, and we now have implants that perform well in a wide range of patients over long periods. However, the new generation of implants is not without flaws, and long-term success remains a concern, particularly in younger, high-demand patients. Hopefully, further advancements in implant technology research would lead to translational clinical applications for better implants.
Antimicrobial strategy implementation for biomaterial implants and devices would be more efficient and impactful if the relationships and alignment of overall translational techniques, procedures, and rules of engagement between the various main collaborators and participants are improved. This would more effectively deliver technological advances to patients and clinicians where they are desperately required.The existing “free form” method for medical product invention, which reacts erratically to various myopic inputs and goals from several different individual partners and players and lacks a robust inventory and alignment of priorities, is neither effective nor productive in resolving these pressing clinical needs to minimise BAI.
Reference
[1] Biomaterials Market – Growth, Trends & Forecasts (2020–2025). https://www.researchandmarkets.com/reports/5175086/ (accessed: October 2020).
[2]ParthaPratim Das, AwasthiAdityaBachchan, RohitSahu, Vijay Chaudhary,Whole body vibration: Effects on human body and role of biomaterials in repairing fracture joints and tissues,Materials Today: Proceedings,Volume 43, Part 1,2021,Pages 141-147,https://doi.org/10.1016/j.matpr.2020.11.250.
[3]Pihl, M, Galli, S, Jimbo, R, Andersson, M. Osseointegration and antibacterial effect of an antimicrobial peptide releasing mesoporoustitania implant. J Biomed Mater Res. 2021; 1– 9..
[4]An, Z., Wu, J., Li, S. H., Chen, S., Lu, F. L., Xu, Z. Y., Sung, H. W., & Li, R. K. (2021). Injectable conductive hydrogel can reduce pacing threshold and enhance efficacy of cardiac pacemaker. .Theranostics, 11(8), 3948–3960.
[5]Marius Fodor, Lucian Fodor &OlimpiuBota (2021) The role of nanomaterials and nanostructured surfaces for improvement of biomaterial peculiarities in vascular surgery: a review, Particulate Science and Technology, DOI: 10.1080/02726351.2021.1871692
[6]Ferrarelli, Fabio and Phillips, Mary L.,Examining and Modulating Neural Circuits in Psychiatric Disorders WithTranscranial Magnetic Stimulation and Electroencephalography: Present Practices and Future Developments, American Journal of Psychiatry, 2021, In-proof, 10.1176/appi.ajp.2020.20071050.
[7]Gallieni, M., De Salvo, C., Lunati, M.E. et al. Continuous glucose monitoring in patients with type 2 diabetes on hemodialysis. ActaDiabetol (2021). https://doi.org/10.1007/s00592-021-01699-6.
[8] Boris Michael Holzapfel, Johannes Christian Reichert, Jan-Thorsten Schantz, UweGbureck, Lars Rackwitz, Ulrich Nöth, Franz Jakob, Maximilian Rudert, Jürgen Groll, Dietmar Werner Hutmacher,How smart do biomaterials need to be? A translational science and clinical point of view,Advanced Drug Delivery Reviews, Volume 65, Issue 4, 2013, Pages 581-603..
[9] David W. Grainger, Henny C. van der Mei, Paul C. Jutte, Jan J.A.M. van den Dungen, Marcus J. Schultz, Bernard F.A.M. van der Laan, Sebastian A.J. Zaat, Henk J. Busscher, Critical factors in the translation of improved antimicrobial strategies for medical implants and devices, Biomaterials, Volume 34, Issue 37, 2013, Pages 9237-9243, https://doi.org/10.1016/j.biomaterials.2013.08.043.



