META-ANALYSIS EVALUATION ON THE EFFECTS OF PSYCHOLOGICAL TREATMENT
November 14, 2019
Network meta-analysis (NMA): A Technique to Synthesize Evidence for Decision Making
December 9, 2019Meta-analysis is a statistical analysis that incorporates several results of scientific research. Meta-analysis can be accomplished when several scientific studies address the same issue, with each individual study record measurements assuming some degree of error.
The goal is then to use statistical methods to extract a pooled approximation nearest to the unk-nown common truth based on the interpretation of this error.
Network meta-analysis compares multiple approaches simultaneously by evaluating experiments that make different correlations in the same analysis. It is a difficult task to decide which one to choose when numerous treatment options are available.
The importance of network meta analysis lies in taking decisions explicitly instead of taking implicitly.
Direct Treatment comparison
Direct treatment comparison refers to the traditional meta-analysis which is performed using research groups that explicitly (head-to-head) evaluate the same two treatments.
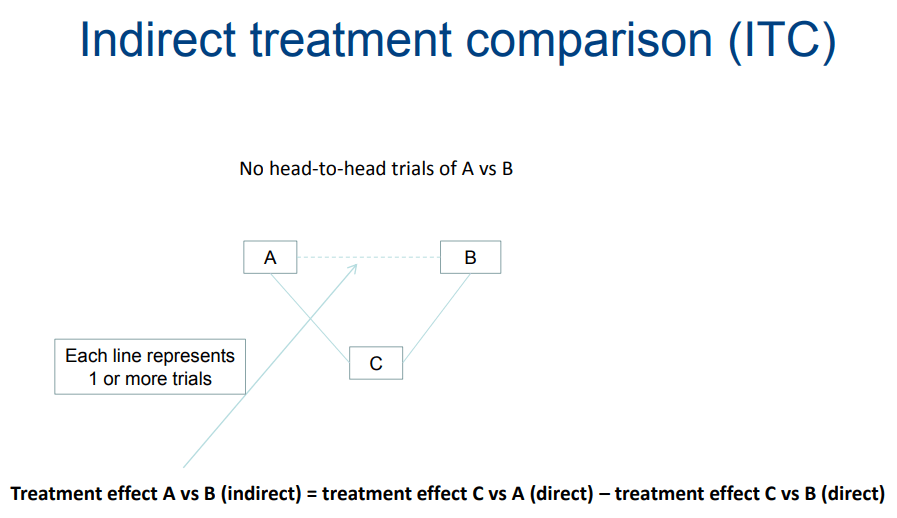
Indirect treatment comparison
It is the comparison of different approaches in health care using data from separate research. Indirect comparison is often used due to the dearth of evidence from head-to-head comparative studies or insufficient evidence.
For example, using indirect treatment comparison; the advantage of A over B is obtained by means of comparing the trails of A vs C to that B vs C, even though this treatment fairly results in inaccurate estimates. Characterization of three or more comparison treatments as a structure of multiple comparison evidence are performed depending on the comparative studies conducted pair-wise.
Over time, available therapies are constantly increasing for many medical indications. For example, for the treatment of depression and other mental disorders, a wide range of different–old and new–medications are available. Physicians, clinicians and health policymakers often have to find out the most cost-effective treatment. Well performed randomized controlled trials provide the most accurate assessments of the comparative efficacy of competing for healthcare approaches. Nevertheless, in randomized controlled trials, most methods were not measured directly. If there is zero or inadequate data from direct comparison trials, the outcomes of different studies may be used to assess the effects of different treatments. Compared to direct comparison within the study, indirect comparison implies a comparison of different procedures between the studies.
Mixed/Multiple treatment meta-analyses
Standard meta-analysis acts as an effective tool for evidence base medicine in spite of its drawback of comparing only two alternative treatments at a time. However, if there are no studies that compare two strategies explicitly, it is impossible to measure their comparative effectiveness.
Multiple treatment meta-analysis therapies integrates both direct and indirect comparison data from a network of trials using multiple procedures to evaluate as widely and precisely as possible the findings of summary treatment.
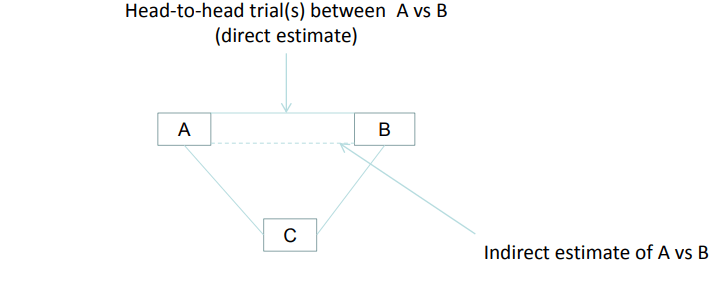
Mixed/Multiple treatment comparison (MTC) is nothing but the generalized form of generic pair meta- analysis for A Vs B to that of data systems. For Example: consider the study on A Vs B, B Vs C and A Vs C. MTC performs two roles where it strengthens the relative effectiveness of two therapies using both direct and indirect comparisons and other one is to select the best treatment by simultaneously allowing all treatments interferences.
Why combine direct and indirect treatment comparison?
Combining direct and indirect may prove beneficial in some cases where we are not confident enough about the results of the head-to-head studies.
· Results are not reliable
· When study quality is low
· Supported by manufacturer
· Research sizes
· A small number of trials.
In addition to that, Network-wide levels of reliability/inconsistency can be helpful and provide continuity across the entire treatment network.
Medical guidelines with NMA
Even though the network meta-analysis has been recently used for development of WHO guidelines, its approach is well known within national agencies for health technology evaluation.
In 2015, GRADE published guidance on how GRADE can be used in combination with network meta-analysis.
In addition, NICE (National Institute for Health and Clinical Excellence) in the UK and Northern Ireland has incorporated recommendations on network meta-analysis within its clinical guidelines manual. Nevertheless, just 8% of 145 NICE clinical guidelines used network meta-analysis in 2012, but possibly the percentage has gone up today.
How to use NMA
It is already mentioned that 8% out of 145 NICE clinical guideline use NMA. There are three types of interventions as depicted by the fig below
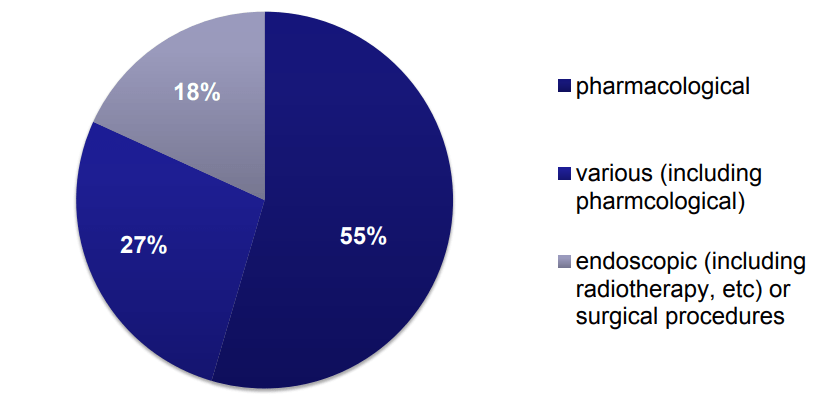
NMA Methodologies followed by NICE clinical guideline
- Comparison of point estimate
- Adjusted indirect comparision
- Bucher’s adjusted indirect comparison
- Bayesian
Advantages of NMA
Network meta-analysis offers numerous advantages to the procedure for developing guidelines.
- One benefit is the opportunity to quantitatively evaluate the comparison of interventions which was not done directly in studies.
This is essential to the development of guidelines as guideline design teams can rely more heavily on expert opinion in the absence of head-to-head. They may, therefore, make comparisons that do not properly account for potential biases in research designs, characteristics of intervention and populations of study.
- A second advantage is that examining both direct and indirect evidence together means stronger evidence base – often to a degree that signifies the difference between grading the strength of evidence as low versus fair or high.
- The third advantage that network meta-analyses for guideline development have is that it provides a parallel analysis of all possible treatment options and makes the use of the available evidence within a single review to the fullest extent.
By doing so it offers a crispier evaluation of the clinical landscape that in effect is better suited for decision-making.
Challenges of NMA
Certain challenges exist while using NMA in guidelines design which includes:
- Network meta-analysis normally synthesizes evidence from only randomized controlled trials. But in the case of randomized controlled trials, hefty cohort studies are not captured. This, however, can’t be called a limitation but it certainly limits the clinical research queries to be answered by network meta-analysis.
- Another challenge is that network meta-analysis models do not mean relative effects, rather they mean than absolute effects.
- Although absolute effects can be derived from relative effects, alternative analytical methods may be preferred. This may be pivotal especially for the evaluation of harmful drugs.
Summary
We can, therefore, conclude that clinical guidelines have growingly become evidence-based with the use of meticulous evidence synthesizing methods. High-quality, in pairs meta-analyses, have been extensively used over the past decades, yet network meta-analysis approaches are gaining more importance to optimize the evaluation of competing interventions. We expect network meta-analysis to be widely used and tailored to establish other guidelines.
Tags:
stastical analysis study | Scientific research | Meta analysis evaluation | network Meta analysis research | randomized controlled trials Bayesian, clinical research | meta-analysis models |