Cohort Study Methodology
A cohort study is a nonexperimental or observational study. The participants in a cohort study do not have the desired result, to begin with. Instead, they are chosen based on the individual's level of exposure. They are then tracked throughout time to look for the occurrence of the desired outcome. Cohort studies include the 1) Framingham Cohort Study, 2) the Swiss HIV Cohort Study, and 3) the Danish Psoriasis and Depression Cohort Study. These studies could be prospective, retrospective, or a combination of the two. Because people do not have outcomes when they enter the cohort study, the timing between exposure and outcome is clearly defined in a cohort design. If the exposure is uncommon, a cohort approach effectively investigates the relationship between exposure and outcomes. A retrospective cohort study may be done quickly and is less expensive than prospective cohort research. In cohort research, participant follow-up is critical, and losses are a significant source of bias in these studies. These investigations are used to calculate the cumulative incidence and rate of occurrence. The longitudinal nature of the data is one of the key advantages of cohort research. Some factors in the data will change over time, while others will not. As a result, complex modelling approaches (such as fixed and random effects models) may be used to analyze these data.
Types of Cohort Studies
Prospective cohort study
In this type of cohort research, all the data are obtained prospectively. The investigator determines who will be included in the cohort. The possible exposure of interest is then measured. The investigator then categorizes the subjects as exposed or unexposed. The investigator then follows these people.
Retrospective cohort study
Data for this type of cohort research are collected from records. As a result, the outcomes have already occurred. Despite the fact that the outcomes have occurred in the past, the core research design remains the same. Thus, the investigator starts with the exposure and other factors at baseline and follow-up and then evaluates the result during the follow-up period.
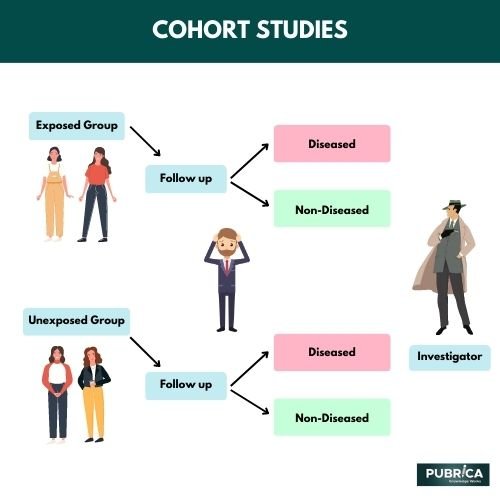
Strengths of a Cohort Study
Limitations of a Cohort Study
Conclusion
In a cohort study, individuals who do not have the result at baseline are tracked over time to determine the incidence of the event. The timing between exposure and consequence is well established in this form of design. The studies might be prospective, retrospective, or a combination of the two. Prospective cohort studies may be time demanding and costly. Losses during follow-up are a significant source of bias in cohort studies; hence, strategies to assure participant follow-up should be included in the design of prospective cohort research. Advanced modelling approaches are essential for analyzing longitudinal data and are favoured in cohort studies. Pubrica's team of researchers and authors create scientific and medical research articles that can serve as invaluable resources for practitioners and authors. Here's how we can assist. We offer a succinct and scientific clinical Literature Review for evidence-based medicine, clinical case reports, meta-analyses, and other similar topics. Recognize the author's specifications to guarantee maximum scientific effect. Create a well-written scientific and academic research paper. Citations (e.g., Oxford, APA, and MLA) should be used when needed. Assistance with all stages of medical and scientific publishing.
Give yourself the Medical edge today
Each order includes
- On-time delivery or your money back
- A fully qualified writer in your subject
- In-depth proofreading by our Quality Control Team
- 100% confidentiality, the work is never re-sold or published
- Standard 7-day amendment period
- A paper written to the standard ordered
- A detailed plagiarism report
- A comprehensive quality report

