How to write a response to the reviewer for your systematic review?
June 20, 2022
The Clinical Case Report: A Review of the Pain Points
September 20, 2022Systematic reviews and meta-analysis are single investigations that aggregate the findings of several other research on a specific topic, such as the efficacy or safety of a medicine or medical device. Systematic Review and Meta-Analysis Services, when completed correctly, are regarded as the most significant evidence on a specific issue and are highly valuable for making health care decisions based on data from several research rather than evidence from the most current or most extensive individual studies.
However, if systematic reviews and meta-analysis are not done correctly or are biased, they may be of limited utility or even deceptive.
Production of systematic reviews on a large scale
Even though methods for conducting systematic reviews and meta-analysis have been around for many decades, they were not widely used in biomedical and health care research until the late 1980s and 1990s, owing to a lack of popular software to create them in large quantities at the time.
In 2003, researchers from the Cochrane Collaboration, a well-known international non-profit organization specializing in systematic reviews and Meta-Analysis Experts, predicted that approximately 10,000 systematic reviews would be required to refuge all clinical trials in health care research. (JPA Ioannidis 2016) However, Ioannidis discovered that from January 1, 1986, to December 4, 2015, almost 59,000 meta-analysis and 267,000 systematic reviews were indexed in MEDLINE. The increase of these publications support has surpassed the rate of development of studies overall: Between 1991 and 2014, yearly publications climbed by more than 2,700% for systematic reviews and 2,600% for meta-analysis, compared to a 153 % annual rise for all MEDLINE-indexed items.
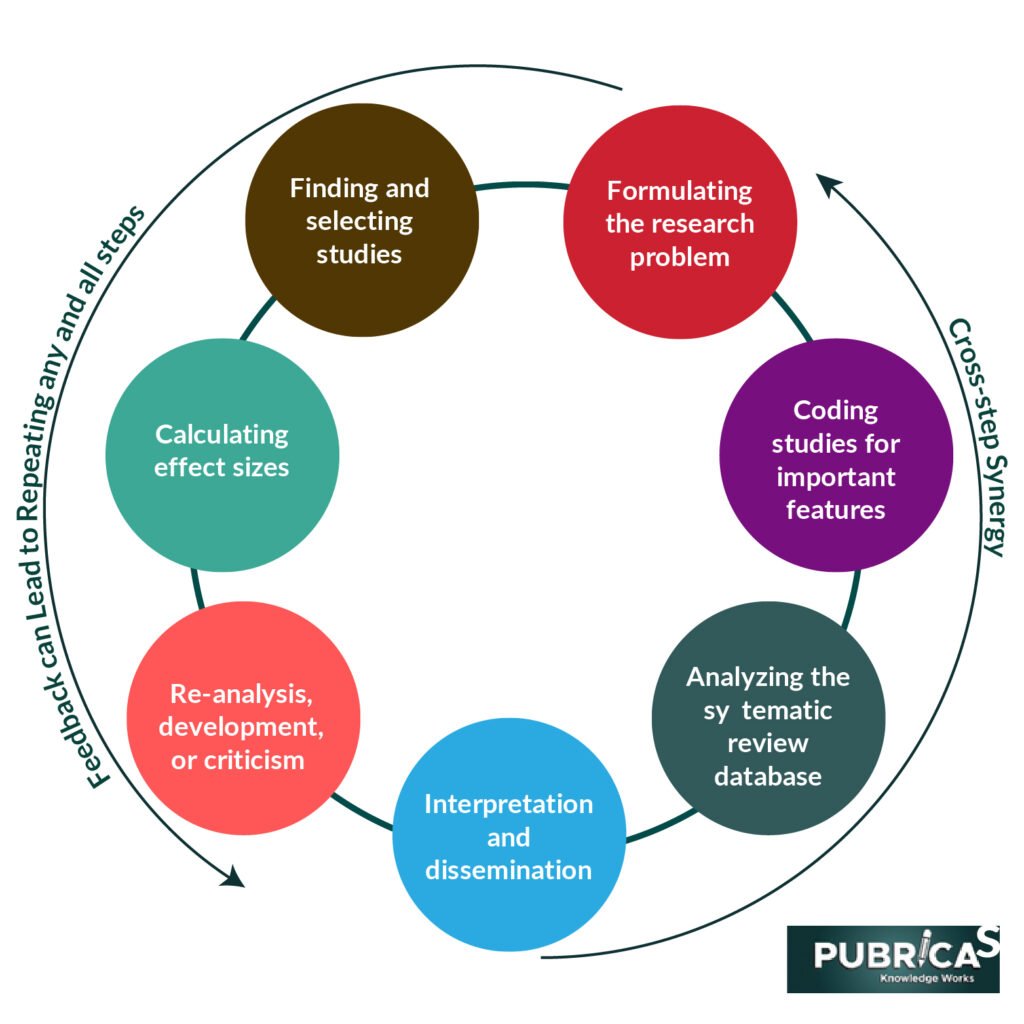
A systematic review and meta-analysis duplication
The increase in writing the systematic reviews and Meta-Analysis services , Manuscript is primarily due to duplication, as most topics have many systematic reviews and meta-analysis. For example, a BMJ review of 73 randomly chosen meta-analysis published in 2010 discovered that for 2/3rd of these studies, there was at least one, and often as many as 13, new meta-analysis published on the same issue by early 2013.
It may be claimed that having many independent writers look at the same data to see if they reach the same results and conclusions or study other outcomes than those included in initial evaluations has some merit. However, according to the aforementioned BMJ study, over a quarter of subsequent meta-analysis were undertaken by some original meta-analysis’ authors, and 65 % of subsequent meta-analysis did not include other outcomes than those included in the original meta-analysis.
Furthermore, overlapping Clinical Meta-Analysis experts might be perplexing since they may not contain the same primary studies that satisfied the criteria for inclusion in the original meta-analysis. While this method may explain why overlapping reviews provide diverse outcomes, readers may find it challenging to reconcile disparate conclusions.
Fragmented evidence
Another critical issue with systematic reviews and meta-analysis is that they frequently attempt to piece together information from much primary research that are intrinsically different without addressing variations in these studies.
Conflicts of interest
Many systematic reviews and meta-analysis are carried out by investigators or contracting companies with ties to the pharmaceutical or medical device businesses. This is a concerning trend since research has shown that industry-sponsored reviews are less open about their procedures and frequently reach conclusions more favorable to the sector than evaluations performed by independent investigators.
Conclusion
Because of the aforementioned problems, which include unneeded, misleading, and contradictory systematic reviews and meta-analysis, this study finds that this defective research does not promote evidence-based medicine and health care. He thinks that just 3% of all meta-analysis are excellent and valuable.
As a result, it calls for a significant overhaul in the production of biomedical research and its credible synthesis, including planning and conducting prospective systematic reviews and meta-analysis without conflicts of interest through collaboration between primary study researchers and those of future systematic reviews and meta-analysis.
These reforms will require the support of many stakeholders, including funders, scientists, medical publications, and consumers. Meanwhile, readers of systematic reviews and meta-analysis should take their conclusions with a grain of salt. Readers should search for any conflicts of interest and see whether other studies found different results or findings.
About Pubrica
Pubrica’s team of researchers and authors create scientific and medical research articles that can serve as an invaluable tool for practitioners and authors. Pubrica medical writers assist you in writing and editing the introduction by presenting the reader with the limitations or gaps in the specified study subject. Our experts understand the structure that follows the broad topic, the problem, and the background before moving on to a narrow topic to state the hypothesis.
References
- Ioannidis J. Next-generation systematic reviews: Prospective meta-analysis, individual-level data, networks and umbrella reviews. Br J Sport Med. 2017; February 21. doi:10.1136/bjsports-2017-097621.
- Page MJ, Shamseer L, Altman DG, et al. Epidemiology and reporting characteristics of systematic reviews of biomedical research: A cross-sectional study. PLOS Med. 2016;13(5):e1002028. doi: 10.1371/journal.pmed.1002028.