Menu
- Services
- Discovery & Intelligence Services
- Publication Support Services
- Sample Work

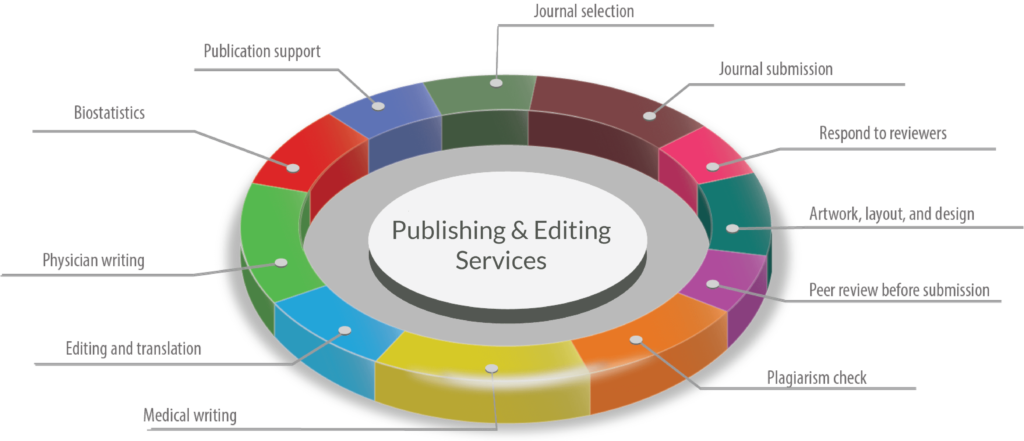
Publication Support Service
- Editing & Translation
-
Editing and Translation Services
- Sample Work

Editing and Translation Service
-
- Research Services
- Sample Work

Research Services
- Physician Writing
- Sample Work

Physician Writing Service
- Statistical Analyses
- Sample Work

Statistical Analyses
- Data Collection
- AI and ML Services
- Medical Writing
- Sample Work

Medical Writing
- Research Impact
- Sample Work

Research Impact
- Medical & Scientific Communication
- Medico Legal Services
- Educational Content
- Industries
- Subjects
- About Us
- Academy
- Insights
- Get in Touch
- Services
- Discovery & Intelligence Services
- Publication Support Services
- Sample Work

Publication Support Service
- Editing & Translation
-
Editing and Translation Services
- Sample Work

Editing and Translation Service
-
- Research Services
- Sample Work

Research Services
- Physician Writing
- Sample Work

Physician Writing Service
- Statistical Analyses
- Sample Work

Statistical Analyses
- Data Collection
- AI and ML Services
- Medical Writing
- Sample Work

Medical Writing
- Research Impact
- Sample Work

Research Impact
- Medical & Scientific Communication
- Medico Legal Services
- Educational Content
- Industries
- Subjects
- About Us
- Academy
- Insights
- Get in Touch

- Services
- Discovery & Intelligence Services
- Publication Support Services
- Sample Work

Publication Support Service
- Editing & Translation
-
Editing and Translation Services
- Sample Work

Editing and Translation Service
-
- Research Services
- Sample Work

Research Services
- Physician Writing
- Sample Work

Physician Writing Service
- Statistical Analyses
- Sample Work

Statistical Analyses
- Data Collection
- AI and ML Services
- Medical Writing
- Sample Work

Medical Writing
- Research Impact
- Sample Work

Research Impact
- Medical & Scientific Communication
- Medico Legal Services
- Educational Content
- Industries
- Subjects
- About Us
- Academy
- Insights
- Get in Touch

Menu
Therapeutic Expertise
Niche areas
- About Us
MEET THE EXPERTS
Clinical research in therapeutics
Outsource or salvage a faltering trial
We offer comprehensive therapeutic expertise across a wide variety of indications. Pubrica Scientific Writing & Publication adapts clinical trial services for specific requirements; we provide complete program development and delivery services for any phase of the trial that you either want to outsource or salvage a faltering trial. Let our experts have their say through new concepts and innovative methods. Our team comprises clinical research associates, project managers, medical supervisors, data management experts, biostatisticians, and medical writers—available at every phase—including trial planning, design, protocol development, safety narratives, in-house data summaries, safety surveillance plan, Case Report Form (CRF), site selection, data analysis, and regulatory submissions. Hence, design cost-effective clinical research trials that deliver data you need to support your novel therapies.
Therapeutic area expertise is particularly important as pharmaceutical and nutraceutical companies develop new classes of compounds that target complex and difficult diseases. Our experts have a say in therapeutic clinical trials; Pubrica Scientific Writing & Publishing has completed holistic projects in the area of CRO therapeutics for pharmaceutical, and biotechnology firms. Our work in therapeutics spans across multiple specializations.
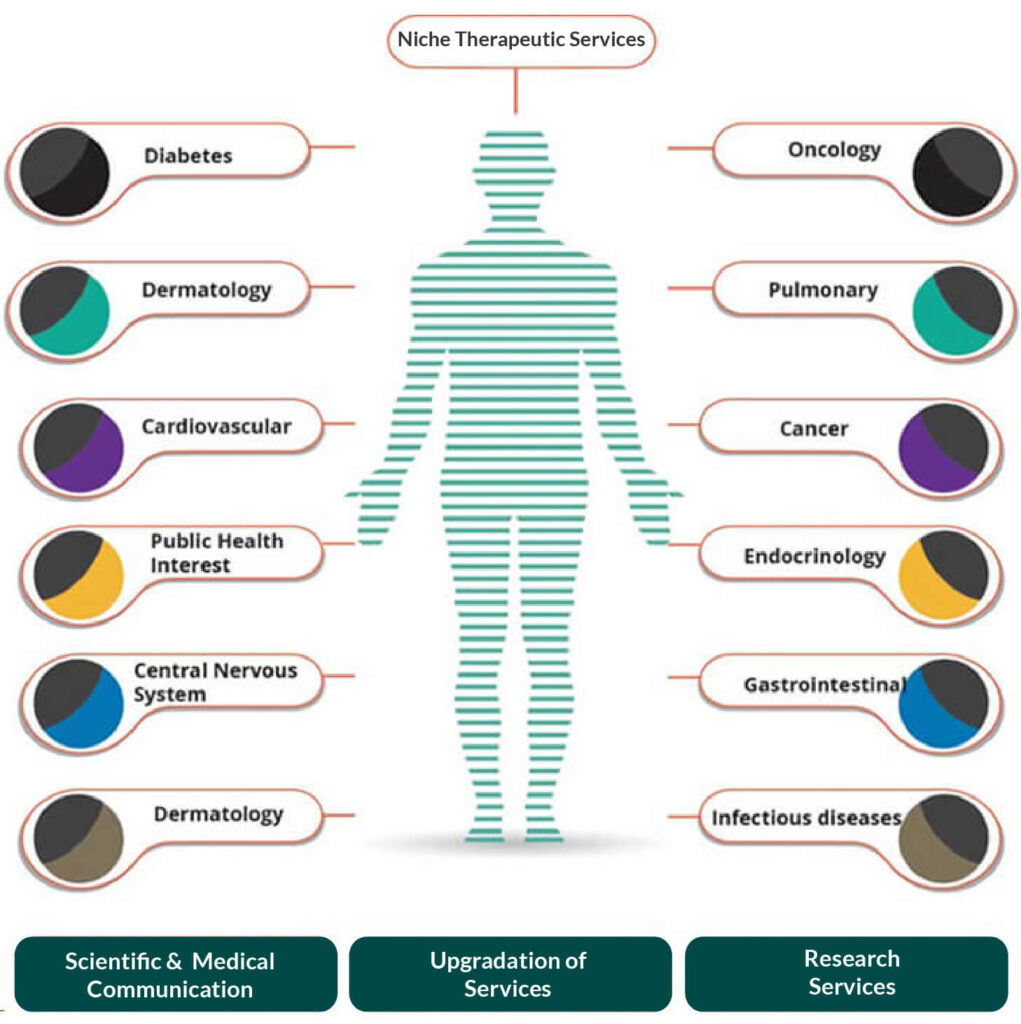
Top areas of therapeutic focus include but not limited to the following list:
- Cardiovascular
- Psychiatry
- Pediatric
- Rheumatology
- Gastroenterology
- Nutritional & metabolic diseases
- Gynecology and obstetrics
Our experts have contributed in phase 1 through phase 4 and also research work in the market. Call us to find out how our CRAs can help you a specific area or if you want to participate in a clinical trial. To know more about our CRO capabilities.
Top Areas of Research
The graph illustrates the percentage of research work in the various therapeutic areas until now. Talk to us for any type of therapeutic support