- Services
- Discovery & Intelligence Services
- Publication Support Services
- Sample Work

Publication Support Service
- Editing & Translation
-
Editing and Translation Services
- Sample Work

Editing and Translation Service
-
- Research Services
- Sample Work

Research Services
- Physician Writing
- Sample Work

Physician Writing Service
- Statistical Analyses
- Sample Work

Statistical Analyses
- Data Collection
- AI and ML Services
- Medical Writing
- Sample Work

Medical Writing
- Research Impact
- Sample Work

Research Impact
- Medical & Scientific Communication
- Medico Legal Services
- Educational Content
- Industries
- Subjects
- About Us
- Academy
- Insights
- Get in Touch
- Services
- Discovery & Intelligence Services
- Publication Support Services
- Sample Work

Publication Support Service
- Editing & Translation
-
Editing and Translation Services
- Sample Work

Editing and Translation Service
-
- Research Services
- Sample Work

Research Services
- Physician Writing
- Sample Work

Physician Writing Service
- Statistical Analyses
- Sample Work

Statistical Analyses
- Data Collection
- AI and ML Services
- Medical Writing
- Sample Work

Medical Writing
- Research Impact
- Sample Work

Research Impact
- Medical & Scientific Communication
- Medico Legal Services
- Educational Content
- Industries
- Subjects
- About Us
- Academy
- Insights
- Get in Touch

- Services
- Discovery & Intelligence Services
- Publication Support Services
- Sample Work

Publication Support Service
- Editing & Translation
-
Editing and Translation Services
- Sample Work

Editing and Translation Service
-
- Research Services
- Sample Work

Research Services
- Physician Writing
- Sample Work

Physician Writing Service
- Statistical Analyses
- Sample Work

Statistical Analyses
- Data Collection
- AI and ML Services
- Medical Writing
- Sample Work

Medical Writing
- Research Impact
- Sample Work

Research Impact
- Medical & Scientific Communication
- Medico Legal Services
- Educational Content
- Industries
- Subjects
- About Us
- Academy
- Insights
- Get in Touch

MOOSE Guidelines For Meta-Analyses Of Observational Studies In Epidemiology
Meta-analyses that combine the results of multiple randomized clinical trials (RCTs) provide the gold standard for scientific evidence. Unfortunately, RCTs are not feasible, practical, or ethical to evaluate interventions for most medical and surgical research topics. Observational studies are the most common type of study design used in clinical research, and nearly two-thirds of published systematic reviews include evidence from cohort, case-control, and cross-sectional studies. These study designs are particularly relied on to evaluate the safety or effectiveness of surgical interventions in real-world practice, identify and summarize rare adverse events, and include more clinically heterogeneous patient populations. While it is estimated that more than 90% of surgical evidence is generated from observational data, the quality of these studies can vary more widely compared to RCTs (1).
These guidelines are directly applicable to systematic reviews. For example, in 2009, the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement was released in several key international journals such as the BMJ, PLoS Medicine, Annals of Internal Medicine and Journal of Clinical Epidemiology. This important step has progressively enhanced the quality of conduct and reporting of systematic reviews. Systematic reviews and meta-analyses have gained importance and are increasingly used as the basis for clinical guideline development. However, conducting a systematic review can be very time-consuming, and the methodology and results must be reported in a structured standardized and transparent manner.
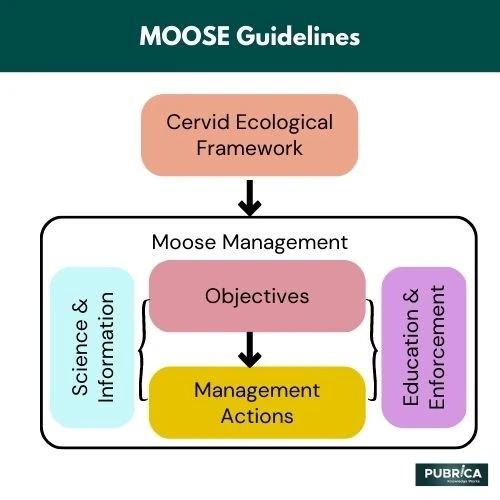
The PRISMA checklist, consisting of 27 items, provides clear guidance to review authors on improving the reporting of their systematic review and meta-analysis. PRISMA is predominantly used for systematic reviews and meta-analyses evaluating RCTs. However, not all evidence can be synthesized from RCTs or in many cases, it is not feasible to conduct RCTs, or they are not available. The results from observational studies can be regarded as complementary to the findings of RCTs and may more accurately reflect the effects in the real world by providing long-term data on interventions’ effectiveness and adverse events. Observational studies can also offer additional advantages over other study designs by identifying and summarizing infrequent adverse events, especially as the number of patients is generally much more significant (mainly when large databases are used). This study design may include a more diverse and clinically heterogeneous patient population and an extended follow-up period (2).
Give yourself the academic edge today
Each order includes
- On-time delivery or your money back
- A fully qualified writer in your subject
- In-depth proofreading by our Quality Control Team
- 100% confidentiality, the work is never re-sold or published
- Standard 7-day amendment period
- A paper written to the standard ordered
- A detailed plagiarism report
- A comprehensive quality report


