Scientific Research Support Services
Pubrica's team of researchers and authors develop Scientific and medical research papers that can act as an indispensable tool to the practitioner/authors. Here is how we help.

Pubrica medical writers follows best practices for research and publication
Our medical Writing & Editing Highlights
The anatomy of a paper
At Pubrica, we follow Introduction, Methods, Results, and Discussion (IMRAD) structure [release by the International Committee of Medical Journal Editors – ICMJE] of scientific papers, which was widely accepted (by over 500 biomedical journals) for retrospective/descriptive & experimental studies.
Consolidated Standards
Depending on your study type, Pubrica Medical writer will determine appropriate reporting guidelines based on EQUATOR Network Checklist. CONSORT statement guidelines for Randomized controlled Clinical trials, STROBE for reporting of observational studies (Cohort, case-control, or cross-sectional studies) or PRISMA statement for systematic review and STARD for diagnostic studies.
Ethical Issues
Clinical reviews are based on comprehensive assessment of wide range of sources of evidence-based medicine. MEDLINE/PubMed Excerpta Refer wide range of libraries, Medica/EMBASE, Scopus, Thomson Reuters Web of Science, the Cochrane Collaboration Database, the Centre for Research Support, TRIP Database, DARE, CINAHL, Google Scholar. We follow COPE guidelines on all aspects of publication ethics.
Literature Review Writing
Preparing a review article can occasionally bring light to a clinical problem that they can be addressed prospectively in a well-designed study.
At Pubrica, we help authors to write a review article that allows a complete analysis of the literature in the trainee’s specific area of scientific interest. Our experts comprehensively cover a specific biomedical topic and justify future research directions. We extensively review and master the literature and then develop some general statements and conclusions with practical implications for patients care. Read More
What You Provide
- Domain area. E.g., Medical, Bio-medical, clinical research
- Area of interest.
- Target Country. E.g. the UK
- Target State, if any or generalized UK population
- Clear Research Proposal - Rough outline
- Suggest 2-3 significant references
- Feasibility of data collection
- University guidelines.
What We Provide
- Extensive Literature survey
- Writing the review based on the outline and organizing content
- Emphasizing main research question
- Clear representation of the present status of the field
- Identifying controversies in the literature
- Base paper references
- Formulating questions that need further research
- Properly referenced document
- A fully formatted document, including line spacing, font, and heading style as per the author's guidelines.
- Proofreading. Examine your document in terms of language errors.
- 300
- 1 Week
Case Report Writing
Preparing a review article can occasionally bring light to a clinical problem that they can be addressed prospectively in a well-designed study.
Case reports are the scientific documentation of a single clinical observation and have a time-honored and rich tradition in medicine and scientific publication. Pubrica has extensive experience in developing a detailed clinical case report that highlights the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Our writers represent a relevant, timely, and essential study design in advancing scientific medical knowledge, especially for rare diseases. Our case study writers of the decision-making process so that other physicians can apply lateral thinking to their cases; moreover, case studies act as instructive examples to people who might encounter similar problems. Read More
What You Provide
- Patient information
- Description conditions
- Category of Your Case Report
- Specified journal
What We Provide
- Detailed case report
- Writing case report based on the outline and organizing content
- Properly referenced document based on journal requirements
- A fully formatted document, including line spacing, font, and heading style as per the author's guidelines.
- Proofreading. Examine your document in terms of language errors.
- 300
- 1 Week
Systematic Review
Preparing a review article can occasionally bring light to a clinical problem that they can be addressed prospectively in a well-designed study.
We follow PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) for randomised controlled trials, and other intervention programs based on Ottawa Hospital Research Institute (OHRI), The STROBE statement (Strengthening the Reporting of Observational Studies in Epidemiology) for cross-sectional, case-control and cohort studies, MOOSE for observational studies in epidemiology, STARD (The Standards for Reporting of Diagnostic Accuracy) or QUADAS for diagnostic accuracy, Cochrane review resources, the SAMPL guidelines for biomedical journals which provide assessment guidelines for fundamental statistical analysis and methods in the literature which are already published. We ensure rigour through methodological checklists such as CASP, AMSTAR, ARIF, etc. Read More
What You Provide
- Research question
- Clearly defining Eligibility criteria
What We Provide
- Protocol Registration
- Refining Eligibility criteria
- Finalizing MESH terms and PICOS
- Flow Chart
- Title, and Abstract Screening: Literature Screening
- Full Text Screening ,Data Selection and Extraction, - Data Synthesis
- Report Preparation – Presentation of Tables and Figures and overall summary
- 1000
- 1 Week
Meta-Analysis
Preparing a review article can occasionally bring light to a clinical problem that they can be addressed prospectively in a well-designed study
Pubrica has extensive experience in conducting meta-analysis a quantitative, formal, epidemiological study design used to systematically evaluate the results of previous research to derive conclusions about that body of research. As a general principle, generating, summarising, and understanding the best available evidence are essential for establishing the benefits and safety of interventions. A well-designed and implemented meta-analysis study can be a great benefit to society. While meta-analysis has the potential to be a powerful tool in evaluating health care treatments and interventions, there are many potential pitfalls and problems that are yet to be resolved. A poorly performed meta-analysis can perpetuate biases from ill-conceived studies or lead to false conclusions. Read More
What You Provide
- Research question
- Clearly defining Eligibility criteria
- Should have completed Systematic Review.
What We Provide
- Ensure that the SR follows the general guidelines of meta-analysis
- Selecting the best statistical model (fixed or random effect statistical models)
- Statistical examination
- Sub-group analyses
- Additional analyses at separate costs
- Report Preparation – Presentation of Tables and Figures and overall summary
- 1500
- 1 Week
Experimental Design
The first-time researcher-author can go a long way toward avoiding design flaws by knowing what the existing literature on the topic says, by seeking advice from Pubrica scientific editors (not only in doing studies but also in getting them published in peer-reviewed journals)
No amount of rewriting, creative data presentation, or statistical manipulation can make up for the fact that the study used the wrong model or study design, collected data in a manner that would not allow a meaningful examination of the hypothesis, or made too few measurements to permit confident conclusions to be drawn. Pubrica medical experts team advice on such things as sample size, unit of analysis, and determinants of both clinical importance and statistical significance. At Pubrica, our team of researchers has a wide range of experience and expertise to develop various forms of the research study based on the objectives. For instance, studies of screening tests, diagnostics, prevention and therapeutic intervention, we use the Randomized Clinical Trial (RCT). There are many instances, however, in which employing the experimental design is difficult or impossible, premature, or unethical. For this reason, a variety of designs—quasi-experimental design, descriptive, and observational designs—are available. Read More
What You Provide
- Domain area. E.g., Medical, Bio-medical, clinical research
- Area of interest.
- Target Country. E.g. the UK
- Target State, if any or generalized UK population
- Clear Research Proposal -
- Suggest 2-3 significant references
- Feasibility of data collection
What We Provide
- Study design
- Methodology
- Sample design
- Experimental procedure
- Statistical procedure
- Ethical
- Data collection
- Experimental design answered all research question
- Proper output
- 400
- 1 Week
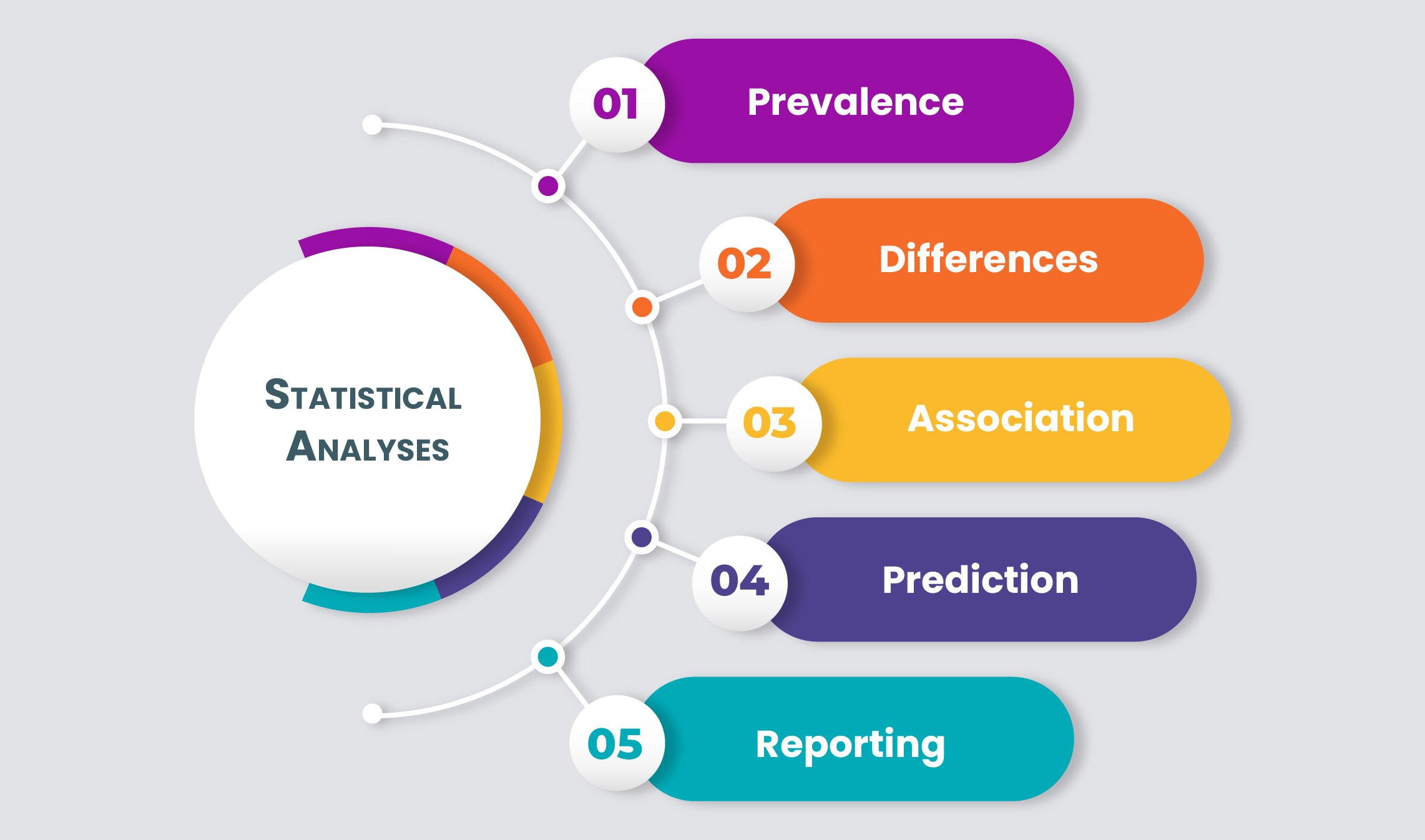
Biostatistics
We provide our researchers and clinical trials investigators with the Information, data management, analysis, and statistical interpretations they require.
Our team protects the privacy and personal data of clinical trial participants. Pubrica experts help you to design questionnaires, create data collection forms, design study protocols, implement study protocols, database management, and web programming. Our team of Statistical programmers, database/web programmers, and data managers have experience in study design, electronic data capture, and subject tracking systems, quality control procedures, and statistical methods. These services are combined and customized to meet your project requirements.
The Pubrica creates systems and procedures to avoid many of the difficulties typically encountered in research collaborations. With an experienced team of data professionals, we can provide data management and procedural consistency to ensure the highest quality data
With the onset of clinical trials and pharmaceutical studies, it has developed in the directions of clinical trial methodology, health outcomes research, survival analysis, longitudinal models and generalized linear models of various types. This typically includes; modern statistical computing, frequentist methods, nonparametrics, likelihood and Bayesian approaches, mathematical statistics, design of experiments, linear models, survival analysis, longitudinal analysis, latent variable models, nonlinear models, categorical data analysis and clinical trials.
Faculty serve on interdisciplinary research teams and provide expertise in statistical methodology, sample size estimations, data analysis, techniques for handling missing data, design of experiments, robust estimation, survival analysis, analysis of microarray data, genomics, and proteomics. Read More
What You Provide
- Domain area. E.g., Medical, Bio-medical, clinical research
- Area of interest.
- Target Country. E.g. the UK
- Target State, if any or generalized UK population
- Clear Research Proposal - Rough outline
- Suggest 2-3 significant references
- Feasibility of data collection
What We Provide
- Design questionnaires
- Data Collection Forms
- Study Protocols
- Database Management
- Programming
Research areas include biostatistics methods and applications, bioinformatics related to cancer, osteoporosis, respiratory and cardiovascular disease, health informatics and data analytics, big data, data capture, management analysis for large clinical trial studies.
Our statisticians have decades of industry experience providing prompt and accurate reports, statistical summaries and efficacy and safety analyses. From the initial stage of study design planning to final stages of data analysis and interpretation.
Our biostatistical expertise includes:
- Statistical consulting for drug and device development
- Clinical trial randomization services
- Sample size calculation and power calculation
- Statistical analysis report writing for clinical study reports and/or manuscripts
- Integrated efficacy and safety summaries
- Data safety monitoring board support and participation
- Multifaceted statistical approaches including pooled analyses and meta-analyses
- Health economics and comparative effectiveness research
- Detailed statistical input for protocols and evaluations
- Detailed statistical analysis plans (SAPs) and table designs
Statistical Programming
Our team provides scalable statistical support. We have highly qualified, seasoned programmers who ensure the flexibility to meet your unique needs and internal guidelines. We can work with your templates or ours, or we can create a set of programming templates specific to your organization.
CDISC Implementation
Pubrica understands how to implement and use the current CDISC standards for data collection and submission. Get solutions to real-world data problems.
Our comprehensive biostatistical services include the following:
- Genomics and genetics
- Brain imaging
- Medicare data
- Clinical repository development
- Data algorithm development
- Programming big data
- Bayesian analysis
- Predictive modelling
- Health economics
- Model interpretation
Our data analytics includes the following:
- Conjoint analysis
- Clinical trial analysis
- Database analytics
- Claims data analysis
- Predictive analytics
Our Clinical trial services includes the following
- Clinical Trial Study Design
- Sample Size Calculation
- Statistical Power Determination
- Statistical Analysis Plan (SAP)
- Development of TFLs
- SAS programming for TFL’s
- SDTM and ADaM compliant datasets
Our biostatistics analysis includes the following:
- Bayesian
- Multiple imputations
- Simulations
- Mixed effects
- Survival analysis
- Multiple comparisons
- Exploratory analysis
Our data visualization tools include the following:
- R
- Hadoop
- Python
- Tableau
- R-Shiny
- Plotly
Data Management & Analysis: Research Advantage
Our team of data management specialists, analysts, programmers and biostatisticians supports the information and analysis needs of researchers throughout Aurora Health Care.
We have three main areas of responsibility:
- Data management: We manage the systems that track people participating in clinical trials. Our oversight ensures patient safety and privacy and project compliance with regulations.
- Information retrieval: Our data experts find the information researchers need to conduct their research and clinical trials.
- Analysis and interpretation: Analysts and biostatisticians analyze and interpret data from research and clinical trials.
Electronic Health Records
Electronic health records store people’s health histories so physicians can understand their patients more fully and make the best treatment choices. For researchers and physicians running clinical trials, these records are important for choosing suitable candidates and evaluating health outcomes after the trial. Our team is responsible for the following:
- Updates: Physicians need to know when their patients are participating in clinical trials for drug interaction and treatment safety reasons. We work with clinical trial coordinators to keep participants’ records consistently up to date.
- Recruitment: We can provide reports to clinical trial coordinators that identify potential candidates for clinical trials based on their health and treatment history. The coordinator will confirm if the patient may be a good candidate. If so, the coordinator will suggest a physician or another clinician inform that person of an upcoming trial and ask if he or she is interested in participating.
- Consent: We track consent information to ensure compliance with the regulations.
- Follow-up data: Our up-to-date system of participants in clinical trials allows researchers to contact people for follow-up evaluations or to recommend another trial for a particular participant.
- 300
- 1 Week
Original Research Manuscript
Preparing a research manuscript article can occasionally bring to light a clinical problem that they can be addressed prospectively in a well-designed study.
We help authors in basic or translational research studies that answer fundamental questions about the basic mechanism or physiology of disease by testing a new hypothesis in in-vivo and in-vitro experiments. Our medical experts have experience in writing scientific manuscripts for clinical studies (RCT), registry studies (population-based studies), and retrospective observational studies. Read More
What You Provide
- Base paper references
- Manuscript title
- Research proposal
- Results
- Appropriate journal for publication
What We Provide
- A list of potential areas
- Background base for the topics
- Base paper references
- Properly referenced document
- A fully formatted document, including line spacing, font, and heading style as per the author's guidelines.
- Proofreading. Examine your document in terms of language errors.
- 300
- 1 Week
Pubrica grant writing services
Preparing grant article can occasionally bring to light a clinical problem that they can be addressed prospectively in a well-designed study.
We prepare a compliant and detailed grant to secure funds; enhance your chances in securing funding with a comprehensive research grant proposal written by Pubrica’s team of writers and editors.
The Research project is expensive to conduct and you need provisions for everything from equipment to technical experts to conference travel and publication charges, acquiring financial support in the form of a grant is pivotal. Once the grant is through, it helps you to broaden the scope of your research or even to form new partnerships and work on new research projects.
Getting research grants in the first place can pose a challenge because the demand is high; the process of writing a grant can be arduous, especially to newbie researchers. Whether you’re applying to the state or federal government, a funding agency, or even your institution, your proposal must be professionally written and sufficiently compelling to convince the reviewer or selection panel to choose you over other suitable applicants.
Pubrica writers know how critical it is to secure funding. We also know how difficult and time-consuming it can be and is exactly why we offer a full detailed grant writing support to take the pressure off. Pubrica can help you with the following
- a) Clear and persuasive proposal
- b) Review and editing of proposal
- c) Checking requirements and compliance
- d) Evaluating the final proposal before submission
Pubrica knows that every business domain is different and comes with its own specific set of requirements. That is why our experts are all industry and subject-area specialists. Talk to Pubrica for specific support in preparing a research grant proposal and securing that fund for your project. Read More
What You Provide
- Research planning
- data and resources
- writing and packaging a proposal
- Submitted research proposal
What We Provide
- Cover Letter
- Executive Summary
- Goals and Objectives
- Methods, Strategies or Program Design
- Evaluation Section
- Information About Your Organization
Other Services
Peer Reviewers from Pubrica
The peer-review process is not just an insurance policy against dissemination of unethical, erroneous, or potentially dangerous material. It also plays a vital role in ensuring that each published article conveys its message as accurately, unambiguously, and convincingly as possible. Peer Reviewers from pubrica are selected for their experience and knowledge of the manuscript’s subject. They donate their time and expertise in reviewing the work as part of the process of science. Their comments are often critical and can sometimes be harsh, but the number of reviews that do not provide ways to improve the manuscript is small. The majority of published manuscripts have been substantially improved as a result of the comments of the reviewers.
Phases of research journey
Define the problem
Select the topic for your research.
Review literature
Acquaint yourself with existing material.
Research design
Select one or more research methods.
Hypothesis
State what you intend to test and the variables.
Interpret results
Implications of the data analysis.
Report findings
State the significance of your findings.
Further research
Follow up with more research after review.
PUBLICATION QUALITY ASSURANCE GUARANTEED


Niche Experts
More than 1000 subject-matter experts. Let our experts call the shots.

Certified Writers
More than 15 years of editorial experience. Leave the writing to us.

Multiple Domains
Served more than 20,000 academic institutions .
Hasten you projects through our experts.
Prolific writers across plethora of areas who know your subject and industry.
Seamless support.
We are with you the whole nine yards of the publishing process.
Frequently asked questions
We are with you the whole nine yards. In this section, we answer the tough questions. For any information, contact us meanwhile, here are some of those queries
We provide a wide variety of services such as succinct & up-to-date scientific clinical Literature Review for an evidence based medicine, clinical case report, meta-analyses, Systematic Review (Prisma), Experimental design, Biostatistics, Original research Manuscript and Grant Writing.
Delivery depends on the order type. However, despite the type of order, if you require literature survey chapter, we will provide extensive and critical writing, identifying controversial in literature, referenced documents, fully formatted document, and assurance of plagiarism. Besides, under the Elite plan, we also link the problem gap with the current literature and provide you with a clear problem statement.
We have Develop a well-written scientific & academic research article, Use appropriate citations (e.g., Oxford, APA, and MLA) as necessary. For more about detailed research area plan selection, please visit
To choose the Research Services, we need clear & precise Domain area. E.g., Medical, Bio-medical, clinical research,Area of interest, Target Country. E.g. the UK, Target State, if any or generalized UK population, Clear Research Proposal - Rough outline, Suggest 2-3 significant references, Feasibility of data collection, University guidelines and also we need following information such as your Qualification, specialization, University, Country, Your experience, possible areas of your interest, Your supervisor capability and university interest, new methodology that is based on related to your Research and area of interest.
Pubrica hires only experienced and certified professionals from European and UK base. All of our medical writers hold Master and PhD degree and have at least five years of writing experience. Each medical writer have their specialization; it helps us to allocate the most appropriate writer according to your discipline. You will get only subject expertise, that’s our assurance, i.e., every order of thesis provide only a relevant research background.
After confirming your order, work will be assigned to Project Associates (PA), who will check the order according to the requirement. The order will, later on, assign to specific subject experts after signing a non-disclosure agreement. She/he will start working on the project as per the agreed deliverables. The order will be delivered after thorough quality check and assurance by the Quality Assurance Department (QAD) and will be given for plagiarism check. After that, you will get the QAD and plagiarism report.
Our work is completely based on your order and requirement. We promise on following guarantees: (1) On-time delivery (2) Plagiarism free and Unique Content (with the acceptability of less than 5-10% plagiarism) (3) Exact match with your requirements (4) Engaging Subject or domain experts for your project. If there is any deviation in the mentioned guarantees, we take 100% responsibility to compensate. However, the quality of work delivered may also get hampered when there is no precise requirement. In that case, you need to take up a fresh order.
We promise on following guarantees: (1) On-time delivery (2) Plagiarism free and Unique Content (with the acceptability of less than 5-10% plagiarism) (3) Exact match with your order requirements (4) Engaging Subject or domain experts for your project. If there is any deviation in the above guarantees, we take 100% responsibility to compensate.
Yes, at Scientific Writing & Publishing Support, our motto is to work hands-on with clients. We guarantee 100% project satisfaction. So we go exceed their expectations. Full-fledged writing services across all domains; moreover, we also provide animation, regulatory writing, medical writing, research, and biostatistical programming services as well. Call us now to get a quote.