Systematic Review Data Repository Plus (SRDR+)
Conducting systematic reviews ("reviews") requires significant time and effort. Making data extracted during reviews openly accessible could have a number of advantages, including decreasing needless repetition of effort, standardizing data, assisting studies to answer secondary research questions, and enabling methodologic research. The Systematic Review Data Repository (SRDR), funded by the US Agency for Healthcare Research and Quality (AHRQ), is a free, web-based, open-source data administration and archival tool for reviews. This article's precise goals are to explain the following:
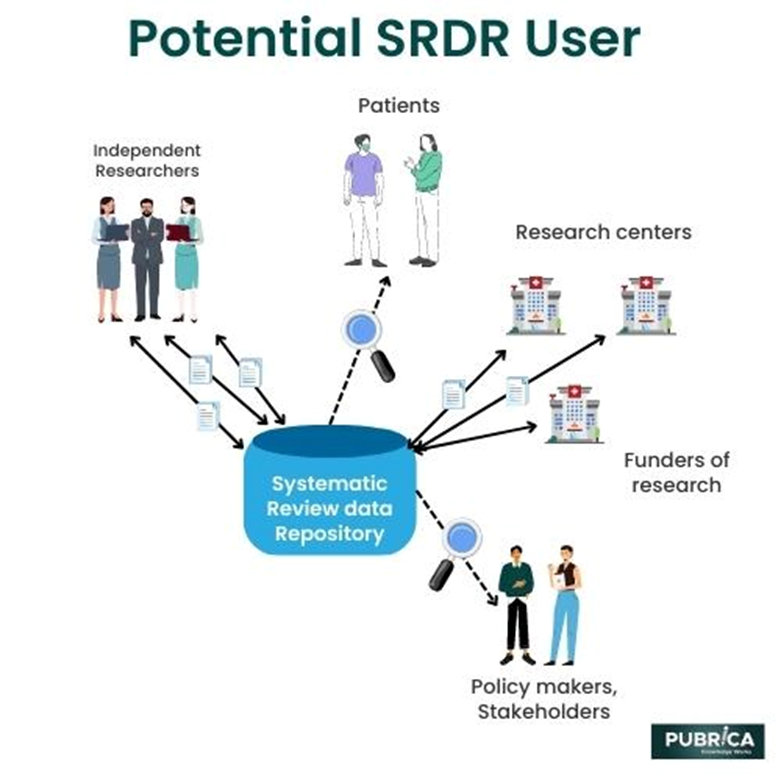
Systematic reviews consume both human capital and monetary resources. Simultaneously, a strong impulse has been to promote open research and data exchange. Technology can help us achieve these goals while also increasing the efficacy of the systematic review business. Data extraction from original papers is an especially inefficient systematic review stage. A robust, user-friendly, and shareable data system can enhance data extraction for both makers and consumers of systematic reviews.
The Brown University EPC released an improved version of SRDR in 2019. SRDR+ is a free, open, collaborative method for extracting and maintaining research data during systematic reviews. To the best of our understanding, this is the only system available for free to anyone anywhere in the world. As a result, SRDR+ is a shared resource. This updated platform's improvements included revamping its core code for easier page loading and saving; facilitating outcome criteria compatible with those on clinicaltrials.gov; and the data comparison tool, which compares and indicates inconsistencies in data extraction. Future paths for SRDR+ include using standards to improve SRDR+ compatibility with external software applications.
SRDR+ is more than a tool for arranging the data extraction procedure in a systematic review. SRDR+ also serves as a data store for previously collected data. The data collected from more than 200 systematic reviews (for more than 20,000 origin studies) had been made available as of February 2022. This implies that future systematic review teams working on similar subjects can save countless hours by reusing these data. This resource is particularly useful for teams performing systematic review updates(1).
Conclusion
Users of systematic reviews, such as guideline developers, policymakers, patients, and the general public, can also obtain study data that may be relevant to their decision-making processes through SRDR+. This data includes summary findings from systematic review initiatives, which can be viewed through SRDR+'s SR360 feature.
Give yourself the Medical edge today
Each order includes
- On-time delivery or your money back
- A fully qualified writer in your subject
- In-depth proofreading by our Quality Control Team
- 100% confidentiality, the work is never re-sold or published
- Standard 7-day amendment period
- A paper written to the standard ordered
- A detailed plagiarism report
- A comprehensive quality report

