Evaluating the Bias Risk in Systematic Reviews of Health Care Interventions
The evaluation of the risk of bias is an essential component of systematic reviews, however, there is no reliable empirical data on the validity of such assessments. In dealing with of such uncertainty, pubrica supports practical guidelines that may be used consistently across review themes, increase process openness and repeatability, and address scientific developments in bias risk assessment.
Introduction
Systematic reviews of health care interventions are essential for synthesizing and summarizing the available evidence on the effectiveness of different treatments. However, the quality of these reviews can be compromised by bias, which can lead to inaccurate or misleading conclusions. Assessing the risk of bias in systematic reviews is therefore crucial for ensuring that the conclusions drawn from these reviews are reliable and trustworthy.
Several key domains of bias should be considered when assessing the risk of bias in systematic reviews. These include selection bias, detection bias, performance bias, attrition bias, reporting bias, and other sources of bias, such as publication bias or funding bias.
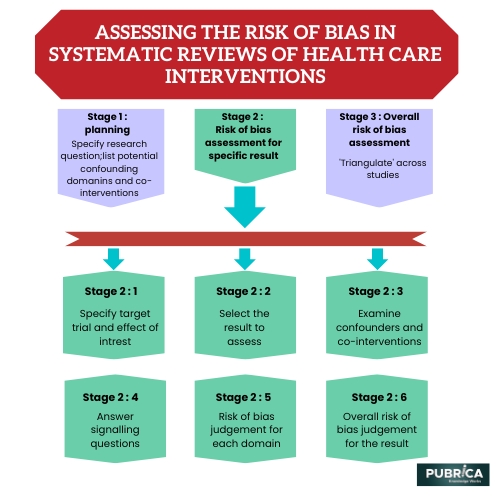
Selection bias occurs when there is a systematic difference between the characteristics of participants in the intervention and control groups. Performance bias occurs when there are differences in the way that the intervention and control groups are treated beyond the intended intervention. Detection bias occurs when there are differences in the way that the outcomes are assessed between the intervention and control groups. Attrition bias occurs when a differential loss to follow-up between the intervention and control groups occurs. Case Reporting bias occurs when the study results are selectively reported or when outcomes are defined post-hoc to produce a desired outcome.
Assessing the risk of bias in systematic reviews typically involves evaluating each study included in the review against a standardized checklist or tool, such as the Cochrane Risk of Bias tool. This involves assessing the study's design and conduct and the likelihood of bias in each domain.
Overall, assessing the risk of bias in systematic reviews is critical for ensuring that the conclusions drawn from these reviews are reliable and accurate. It is important to use standardized tools and checklists to evaluate the risk of bias and to consider all relevant domains of bias when making these assessments.
Conclusion
The risk of bias assessment is an important stage in conducting systematic reviews since it influences the study's subsequent procedures and decisions. It also plays a vital part in determining the strength of the evidence. Research services provided by Pubrica The relevance of risk of bias assessment to the overall systematic review job necessitates that assessment methodologies be founded on theoretical concepts, at the very least, and solid empirical evidence where available. EPCs should stress openness of judgment in assessing the possibility of bias in research by carefully documenting procedures and judgments.
Give yourself the Medical edge today
Each order includes
- On-time delivery or your money back
- A fully qualified writer in your subject
- In-depth proofreading by our Quality Control Team
- 100% confidentiality, the work is never re-sold or published
- Standard 7-day amendment period
- A paper written to the standard ordered
- A detailed plagiarism report
- A comprehensive quality report

