
Scientific and Academic E Poster Creation 7 Essentials for Researchers
February 1, 2021
Critical Checklists for Supporting Scientific Publication Journey
February 8, 2021In-Brief:
- Owing to the significance of safe use of medicines, adverse drug reaction (ADR) monitoring has become an essential component to be achieved along with other health-care facilities from Pharmacovigilance Literature Screening Services.
- Pharmacist being drug professionals are in an excellent position to offer professional assistance for ADR organization.
- Pubrica explains the recent trends, challenges, and future prospects in Pharmacovigilance’s literature surveillance using literature screening pharmacovigilance and provides a literature review writing service.
Introduction:
The field of drug protection has been receiving a great deal of attention lately. Almost weekly, tabloids and scientific journals, publish articles on drugs that cause unexpected adverse drug reactions (ADRs). These articles have the unfortunate result of evoking apprehension in both patients and health professionals regarding using these drugs from literature search services. Amore serious consequence may be that the patient stops taking the prescribed medication, which may lead to an even more difficult situation than the ADR he was initially concerned. Pharmacovigilance, defined by the World Health Organization (WHO) as ‘the science and activities connecting to the detection, assessment, understanding and anticipation of adverse effects or any other drug-related problem’, plays a vital role in confirming that doctors, together with the patient, have enough information to make an educated decision when it comes to picking a drug for the treatment using Literature review services.
Recent trends in Pharmacovigilance:
Moving to drive operational Efficiency
Specific re-appropriating in Pharmacovigilance is turning into a broadly utilized way to deal with adapting to the developing expenses of keeping a profoundly qualified and prepared pharmacovigilance team in-house using literature review service for Pharmacovigilance.
For Manufacturers and Sponsors, a very much actualized pharmacovigilance reevaluating program brings observable advantages including:
- Reduced fixed expenses;
- Increased adaptability;
- Better results in the short-and long haul
These days an ever-increasing number of organizations reevaluate their pharmacovigilance errands to accomplish better administrative consistence, more significant, better profitability, and improved vital choices from medical literature monitoring services.
Information Analytics to Drive Actionable Insights
According to a clinical literature review, the successful administration of health information put away across numerous stages is imperative for away from security occasions. The developing number of Life Sciences organizations goes to cutting edge logical methods in Pharmacovigilance to look at huge and changed informational collections that contain health data. They endeavour to uncover new examples, obscure connections, patterns, and patient inclinations that help them guarantee patients’ security all the more viably. These days, pharmacovigilance examination gives a genuine chance to outfit information adequately, guarantee administrative consistence and drive unique experiences.
Big Data to Protect and Assimilate Huge Amount of Information
As of late novel wellsprings of actual proof and trial information in the mechanical structure, they have also opened up to pharmacovigilance experts.
In Pharmacovigilance, enormous information incorporates such sources as:
- Signal discovery;
- Substantiation and approval of medication or immunization health signals;
- Online channels and web-based media.
Because of its intricacy, Big information address both a chance and a challenge. With the help of innovation arrangements with cutting-edge figuring capacities, Life Sciences organizations utilize enormous information to screen and study drug health more successfully.
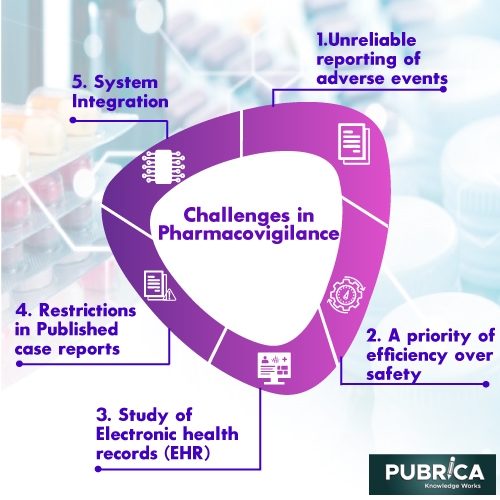
Challenges in Pharmacovigilance:
Unreliable reporting of adverse events
The event of an unfavourable occasion isn’t continually during a visit to the Healthcare Center. It can happen following a few hours of managing the medication. Patients neglect to recall all the applicable data about unfriendly occasions and can’t report it precisely. Patients are on edge and report all their inconvenience as antagonistic occasions. Cold medication occasions (ADE) revealed are not generally genuine and might be manifestations of an illness. Different episodes where a patient has not adhered to guidelines during prescription or patient has had results brought about by accompanying medications brought with the examination medication could be accounted for as unfriendly occasions. Such off-base detailing can lead the medication health councils to wrong ends, leading to the suspension or withdrawal of drugs.
A priority of Efficiency over safety
More modest medication organizations may focus on viability over health in clinical preliminaries prompting a trade-off in medication quality. A couple of supporters don’t use the sign identification to identify and successfully settle the issues reasonably. Medication advancement depends on adjusting viability and health similarly.
Study of Electronic health records (EHR)
EHR gives an incredible abundance of data about constant and certifiable drug use. A couple of restrictions incorporate the unstructured story data that is convoluted to investigate. There might be not many EHR cases to examine a specific medication, yet various issues are needed to produce a sign. Another test is the absence of admittance to clinical records because of patient security Systems.
Restrictions in Published case reports
Reports in clinical diaries about the speculated antagonistic impacts are a setup approach to caution about medication risks. These reports are one of the signs producing reports open by everybody.
System Integration
Reconciliation between the different systems, for example, the clinical preliminary administration System (CTMS), clinical information the executive’s System (CDMS), item execution System, clinical coding application, and CRO Systems is urgent for pooled information investigation. They normalize the clinical areas, signal definitions, unfavourable occasions, and clinical guarantee quality sign investigation. Normalization is a test as there is no standard structure to permit System reconciliation. Even though the default record design XML concurs it isn’t executed. Hence clinical information is gathered by the current support in discrete EDC or using paper-based case report structures.
Future Prospects in Pharmacovigilance:
On an administrative level, progress has been made during the previous few years. Be that as it may, the significances of these progressions still can’t seem to get noticeable. Like this, it has not yet been demonstrated if these advancements have added to better pharmacovigilance lead. To show Pharmacovigilance as a science, it is fundamental that the scholarly community grows new techniques to fortify the current System. Little accentuation has been placed into creating data to help a medical care proficient or a patient utilize medication’s dynamic interaction. The social affair and correspondence of this data is a significant objective of Pharmacovigilance.
Active reconnaissance is essential to get data about the security of medication at the beginning phase. When growing new strategies for dynamic post-promoting observation, one needs to remember the significance of having the option to assemble data reasonably. Unconstrained detailing has for sure been demonstrated to be a helpful instrument in producing signals. Yet, the moderately low number of reports for a particular affiliation makes it less valuable in distinguishing tolerant qualities and danger factors that will add to an ADR in someone in particular. This data is fundamental regarding a medical care supplier suggesting whether a specific patient should utilize the medication being referred.
When confronting an ADR, questions that patients just as the treating doctor can ask are: will this ADR vanish? What amount of interval will it require before it does?; what treatment is needed? None of the fundamental techniques utilized today in post-showcasing reconnaissance can give a response to these inquiries. In this manner, it is critical to creating strategies that can follow a patient using a specific medication over the long haul. The data assembled using such techniques will empower such inquiries to be replied. Pharmacogenetics could assume a part in distinguishing singular danger factors for the event of specific ADRs
Conclusion:
The growing complexity in Pharmacovigilance services leads to outsourcing PV as a whole or part of it. Pharma industry is still leveraging 10+ years old legacy systems to monitor safety and drug misuse. The technical advancement such as Cloud-based solutions, Mobile health devices, Artificial Intelligence, Blockchain, and Machine learning will improve PV’s effectiveness and enhance the efficacy of drugs. Pubrica also explains the recent trends, challenges, and future prospects in Pharmacovigilance’s literature surveillance along with scientific literature search services
References:
- Dal Pan, G. J. (2014). Ongoing challenges in Pharmacovigilance. Drug safety, 37(1), 1-8.
- Inácio, P., Cavaco, A., & Airaksinen, M. (2018). Current trends in Pharmacovigilance: value and gaps of patient reporting. International journal of clinical pharmacy, 40(4), 754-757.
- Härmark, L., & Van Grootheest, A. C. (2008). Pharmacovigilance: methods, recent developments and future perspectives. European journal of clinical pharmacology, 64(8), 743-752.
