
The Role of Packaging Design In Drug Development
May 6, 2021
What is the Bayesian random-effects meta-analysis model for normal data
May 26, 2021In Brief
Cost analysis of implementing evidence-based analysis has become important within implementation science and is critical for bridging the research to practice gap to improve access to quality healthcare services. Costing studies in this area are rare but necessary since the cost can be a barrier to implementing and sustaining evidence-based analysis (1).
Introduction
To provide physicians with a foundational view of health economics studies, such as cost-benefit, cost-effectiveness, and cost-utility analysis. The procurement of services that yield the best patient benefit at the lowest cost is a challenge in a healthcare economy with occasional funding. To make informed decisions in value-based care, information on benefits and costs is needed. Any cost-benefit, cost-effectiveness, or cost-utility model relies heavily on costs.
Estimating model costs is difficult and can vary greatly based on the viewpoint of the stakeholder. First, the investigator must decide which costs should be included in the report. In economic terms, an expense is any capital use. Both prices must be in the same units for study, which is normally currency. Time and materials are all categories of costs that must be valued and factored into the decision-making process (2).
Evidence-based analyses to look cost-effectiveness at the FDA medical devices
Mainly FDA’s Center for Devices and Radiological Health (CDRH) is in charge of medical technology premarket evaluation, strong manufacturing process standards, and post-market surveillance. However, the FDA’s premarket appraisal of most medical devices is not as stringent as it is for medications until they are introduced to the market. While all new drugs must go through rigorous premarketing tests in randomized clinical trials before receiving FDA approval, only a few new devices must go through the same process. Instead, the FDA categorizes new devices into low risk, moderate risk, and high risk(3). Low-risk products (bandages, splints, and surgical drapes) account for half of all medical devices sold each year, and they are exempt from all premarket review criteria.
Current Economic Considerations in FDA’s Drug and Medical Device Approval Processes
The FDA does not use economic conditions in its medication and medical device approval procedures. The Food, Medicine, and Cosmetic Act, the Agency’s principal legislative jurisdiction, neither authorizes nor forbids the use of financial requirements in the FDA’s assessment of applicant drugs and products. The legality of using cost efficacy to assist with evaluating experimental medications and products is yet to be determined (4).
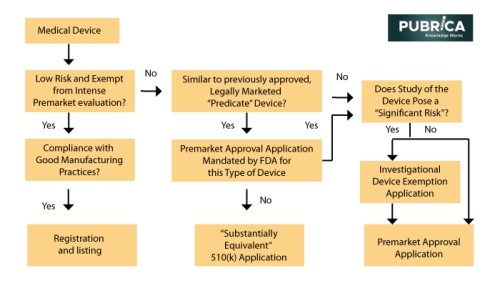
The Economic evaluation and Clinical data from RT – Device manufacturers
In the management and care of cancer, RT plays a crucial part. External beam radiotherapy (EBRT) and its innovative and localized cancer treatment capabilities, such as stereotactic body ablative radiotherapy (SABR) and proton therapy, are the subject of many studies. Internal radiotherapy (IRT) employs invasive surgical techniques and seed implementation (brachytherapy), in which nuclear seeds are directly inserted next to cancerous cells for invasive procedures.
The figure depicts the traditional EBRT pathway, which includes the following steps: (1) immobilization and visualization of the patient using modalities such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission computed tomography (SPECT), ultrasound, or plain x-ray radiographs; (2), (3), then tumour segmentation, in which attending clinicians determine tumour volume parameters (such as form and location) and margins when taking into account essential structures before image validation procedures; (4) Physicians and physicists then use computerized treatment planning systems (TPS) to digitally replicate the radiotherapy procedure, where dose beam directions and intensities are optimized for improved patient treatment.
The optimization may be performed by the operator (planning), who chooses the beam number, form, paths, and dosage contribution, or by a computer algorithm (inverse treatment planning). Once the treatment arrangements have been finalized and accepted, the actual patient irradiation procedure begins, in which (5) the entire dose is separated into small doses (fractions) and administered to the patient at predetermined times (usually daily) to enable healthy tissues to recover between procedures.
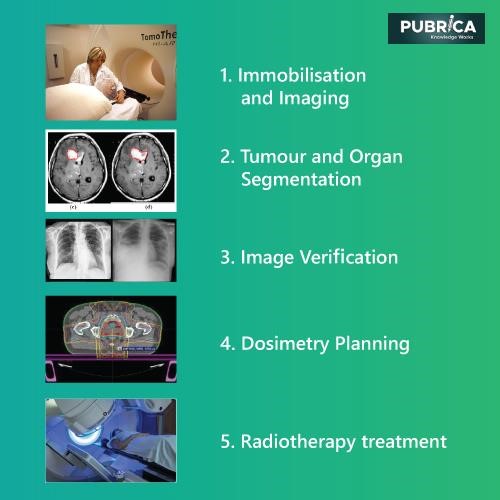
Treatment planning process
TPS-based computer algorithms are used in the care preparation process to assess the best treatment parameters for the individual’s condition. Goal volume(s), dose-limiting mechanisms, dose allocation, dose fractionation, dose delivery, patient positioning, therapy machine configurations, and adjuvant therapies are among these criteria. In addition, the device generates reference images and other data that help in the patient’s set-up and location verification of treatment fraction. Over several weeks, attending clinicians safely monitor the process’s final performance.
Quality assurance (QA) is an important aspect of radiotherapy treatment because of the heavy and extremely harmful doses of radiation administered to patients. The value of high-quality delivery has been shown in many clinical research, with scientific QA generally measuring both the dosage administered and the geometric accuracy of the delivery. The confirmation of a procedure in a standardized phantom or the verification of dose administered in a delivery series for a particular patient are two typical QA processes in radiotherapy delivery. The issue with both is that they almost all use a generic description of a human patient (5).
Conclusion
The medical device industry spends a lot of money on emerging technologies, testing, product creation, insurance, and patient access. Near working partnerships with suppliers will help align priorities by providing access to hospital management and buying teams to inform them on the effect of technological investments on cost-benefit analysis and better patient outcomes.
Hospitals and physicians should also take the lead in developing health performance reporting and benchmarking procedures and disseminating the data needed for evidence-based technology evaluations. More sophisticated information systems that connect goods to cost, results and protection are being established. Still, widespread implementation would necessitate establishing criteria for comprehensive data collection and specially trained personnel to perform evidence-based research that includes cost-benefit and cost-effectiveness analysis (6).
References:
- Okafor, Charles E. “Management of Chest Indrawing Pneumonia in Children Under Five Years at the Outpatient Health Facilities in Nigeria: An Economic Evaluation.” Applied Health Economics and Health Policy 19.3 (2021): 429-437.
- García-Álvarez, David, Núria Sempere-Rubio, and Raquel Faubel. “Economic Evaluation in Neurological Physiotherapy: A Systematic Review.” Brain Sciences 11.2 (2021): 265.
- Tonin, Fernanda S., et al. “Principles of pharmacoeconomic analysis: the case of pharmacist-led interventions.” Pharmacy Practice (Granada) 19.1 (2021).
- Okafor, Charles E. “Management of Chest Indrawing Pneumonia in Children Under Five Years at the Outpatient Health Facilities in Nigeria: An Economic Evaluation.” Applied Health Economics and Health Policy 19.3 (2021): 429-437.
- Rance Tino et al. 2020 Int. J. Extrem. Manuf. 2 012003
- Thompson, Ryan, Jorge Martin Del Campo, and Dagna Constenla. “A review of the economic evidence of Aedes-borne arboviruses and Aedes-borne arboviral disease prevention and control strategies.” Expert review of vaccines 19.2 (2020): 143-162.
