Using R Programming to Analyze Clinical Trial Data
R programming has not been widely used in clinical studies. Or prominent despite its recent growth over the past few years. Its actual use appears limited by several problems, some of which are due to misconceptions (for example, validation), but others are related to a lack of understanding of its potential. Despite these constraints, R is undoubtedly building out a (growing) niche in the pharmaceutical business.
There is frequently a knowledge gap between developed statistical methods and their implementations in biostatistical research and clinical trials. Clinical Trial Data Analysis Using R fills this need by providing a complete description of biostatistical analyses of clinical trial data and step-by-step instructions on using R to perform the statistical approaches.
Current Trends of R in Pharma and CROs:
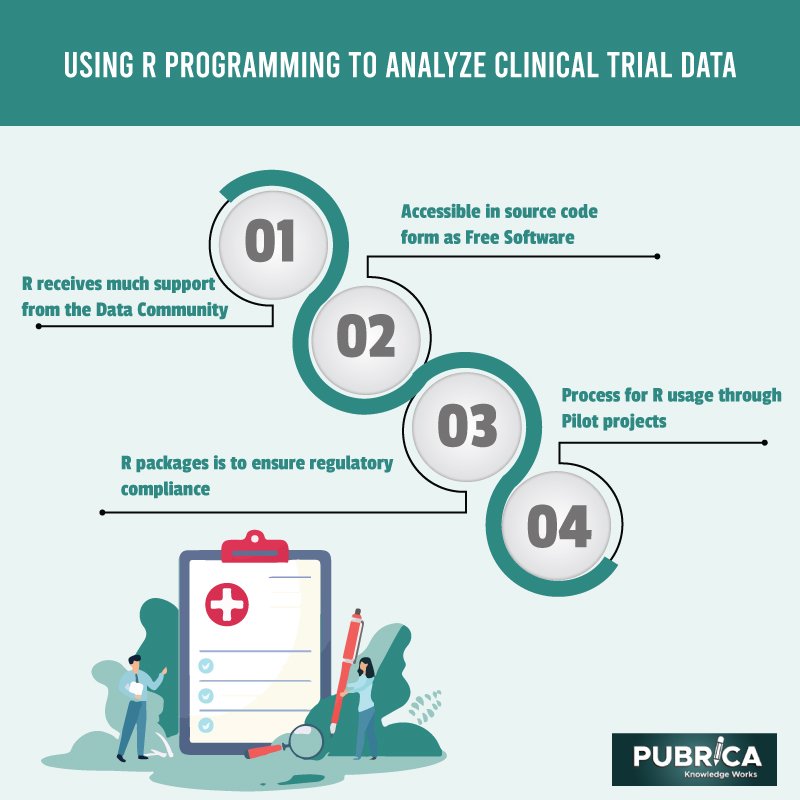
R is a statistical computing and graphics language and setting. It is accessible in source code form as Free Software under the Free Software Foundation’s GNU General Public License. R receives much support from the Data Community since it is open-source software. The availability of source code allows for improved and extensive documentation. R has almost 2 million users all across the world. There have been a few Early Adopters of R, and they have had some difficulties. One of the most prevalent uses of R packages is to ensure regulatory compliance. If R is to be considered for work connected to regulatory filings, risk assessments of R packages, feasibility analyses, and process for R usage through Pilot projects with the relevant paperwork must all be completed.
Author’s Update: Keep up to date on industry advancements, support, and training.
Pubrica Connect: Read articles about research, technology, and health communities daily.
Researcher Academy:Improve your manuscript by learning academic writing skills.
Language editing by Pubrica Author Services:Before submitting your work, double-check that it is written in proper English.
Translation by Pubrica Author Services: Translate your work into English professionally.
Search engine optimization (SEO): Make your article more visible by using SEO.
Your paper, your way: Save time by making your first submission simple.
