
Preparation of Article Based on the Study Protocol
July 5, 2021
How to conduct abstract screening for systemic review- From Beginning to End
July 12, 2021In brief
Even though there have been many systematic reviews of implantable medical devices published, no empirical evaluation of the reviews has been done. To understand the methodology utilized, identify existing strengths, limitations, flaws, and specific issues, and provide recommendations to enhance future conduct and reporting, we undertook a critical appraisal of the quality of reporting in systematic reviews of implanted medical devices. These characteristics may have an impact on the study outcomes (1).
Introduction
In medical practice and research, systematic reviews service have a well-established place. Clinicians utilize systematic reviews to stay up to date on current research and compare the efficacy of competing therapies. Although many systematic evaluations of medical devices have been published, no empirical evaluation of the reviews has been conducted. The findings of an empirical evaluation of systematic reviews of medical devices could be utilized to design new reporting rules and improve the conduct and quality of systematic reviews of medical devices reporting (2).
The Consolidated Standards of Reporting Trials (CONSORT) Statement was recently updated to include trials of non-pharmacological treatments; however, there are still no recommendations for systematic evaluation of medical devices. Implantable medical devices are one big group of devices of interest. According to the Food and Drug Administration (FDA), these are devices that are partially or implanted into the body or a natural orifice via surgical or medical procedures and are intended to remain in the body or orifice for at least 30 days, according to the Food and Drug Administration (FDA) (or permanently). Such devices can only be physically removed or medically deactivated.Implantable devices can also be utilized to replace an epithelium or ocular surface. The market for implantable medical devices in the United States is expected to expand 8.3% year to $49 billion, with spinal implants, cardiac implants, and orthobiologics being the fastest-growing categories (substances that accelerate healing of injured bones).
Here assumed a critical appraisal of the quality of reporting in systematic reviews of implanted medical devices as the Evidence-based Practice Center (EPC) designated for the crosscutting concentration of diagnostic testing, imaging technologies, and medical and assistive devices. The project’s objectives were to assess published systematic reviews and meta-analyses to understand better the methodologies used and identify current strengths, limitations, deficiencies, and unique challenges and make recommendations for future conduct and reporting. They chose to focus on five broad categories of implantable medical devices based on the recommendations of the Technical Expert Panel (TEP) and to ensure inclusion of the most commonly used and expensive devices: cardiac implantable devices, vascular interventional devices, orthopaedic implants, skin-replacement grafts, and neurostimulators(3).
Defining active implantable medical devices
An active medical device operates by using and converting a large amount of energy. Except for gravitational and direct human energies, active devices can use any energy. Active medical devices, as defined by the Therapeutic Goods (Medical Devices) Regulations 2002, can be broadly classified into two categories:
- The manufacturer intends for active medical devices for diagnosis to be used on a human being to provide information for detecting, diagnosing, monitoring, or treating physiological conditions, states of health, illnesses, or congenital deformities, either alone or in combination with another medical device.
- The manufacturer intends for active medical devices for therapy to be used on a human being to maintenance, modify, replace, or restore biological functions or structures to treat or alleviate an illness, injury, or handicap, either alone or in combination with another medical device(4).
Literature search
Systematic reviews published between January 2009 and December 2010 were found using keywords for each of the five categories of implantable medical devices in MEDLINE® and the Cochrane Database of Systematic Reviews. Articles with abstracts that described searches or eligibility criteria for study identification or included terms like “systematic,” “evidence,” “evidence-based,” “meta-analysis,” or “pooled analysis” were considered potentially relevant reviews.
Eligibility criteria and citation screening
Many published systematic reviews of implantable medical devices did not explicitly report all three fundamental components during full-text screening.
Because the goal was to assess reporting characteristics, it included reviews of any implantable device from any of the five categories that looked at a recent publication. For example, a review of a pacemaker, a review of a defibrillator, or a review of both a pacemaker and a defibrillator might be included in the cardiac implantable device category. Systematic reviews of any type (randomized trials, non-randomized comparative studies, or observational research), as well as synthesis technique, were considered (qualitative or quantitative synthesis including meta-analyses of individual patient data) (5).
Table: 1 Reporting items for systematic reviews of implantable medical devices
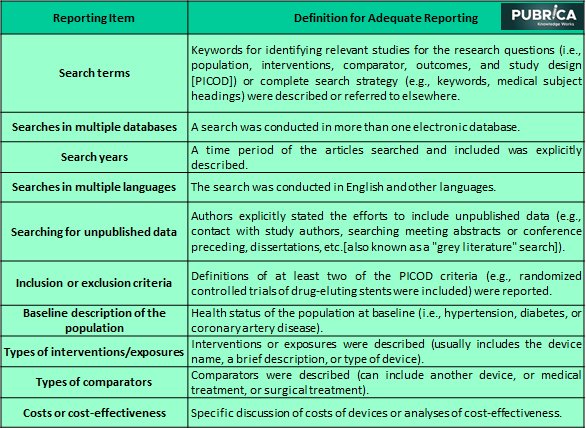
Data extraction
There are currently no defined techniques or protocols for assessing the quality of systematic reviews of implantable medical devices reporting. Here examined the TEP to identify device- and operator-specific information relevant to these devices’ evaluation. In addition to the 30 systematic reviews, specific information items described in the MOOSE and PRISMA recommendations have found eight device- and operator-specific information items.
Recommendations of reporting items for systemic reviews of implantable medical devices
Reporting of device or procedure-specific data
PROSPERO—the International Prospective Register of Systematic Reviews—also offers systematic review reporting, conduct, scientific writing, and publication through a formal protocol registration process. At the time of registration of systematic review protocols, propose include eight new device- and operator-specific elements unique to implanted medical device research. This project would encourage academics to report systematic reviews of implantable medical devices more accurately and transparently. Also, propose adding eight new device- and operator-specific topics to the extension guide unique to implantable medical device studies (6).

Cardiac defibrillators with or without pacemakers:
- Device type
- Method of implantation
- Position of the electrode
- Description of microprocessor technology and programmable features
- Alert features that monitor lead impedance
Vascular interventional devices (e.g., stents)
- Type of stent and stenting technique
- Generation of the stent (e.g., first or second generation)
- Type of antiproliferative drug used
- Delivery system
- Polymer layer
- Stent frame
Orthopaedic implants
- Type of device
- Surgical technique or approach
- Number and location of devices
- Fixation and supplementary materials such as plates and screws
- Type of device coating
Skin-replacement grafts
- Type of skin graft required
- Composition of graft
- Graft type: bioabsorbable or requiring removal
Neurostimulators
- Stimulation parameters
- frequency
- intensity
- pulse width
- Electrode location
Conclusion
The lack of reporting of some essential general items applicable to any systematic review and device- and operator-specific information is revealed in a review of systematic reviews on implantable medical devices. Eight device- or operator-specific items were also identified as potentially useful in reporting on systematic reviews of implanted devices and might be included in reporting recommendations.In recent years, the number of systematic evaluations of implantable medical devices has increased dramatically, with reviews appearing in a wide range of journals. Failure to include data on procedures and devices could lead to incorrect synthesis or interpretation of results because there is no widely acknowledged standard for reporting information to implantable medical device research(7).
References
- Reckers-Droog, Vivian, et al. “Challenges with coverage with evidence development schemes for medical devices: A systematic review.” Health Policy and Technology 9.2 (2020): 146-156.
- Fraser, Alan G., et al. “Implementing the new European Regulations on medical devices—clinical responsibilities for evidence-based practice: a report from the Regulatory Affairs Committee of the European Society of Cardiology.” European heart journal 41.27 (2020): 2589-2596.
- Baman, Jayson R., et al. “Management of systemic fungal infections in the presence of a cardiac implantable electronic device: A systematic review.” Pacing and Clinical Electrophysiology 44.1 (2021): 159-166.
- Lin, Andrew Y., et al. “Safety and efficacy of cardiovascular implantable electronic device extraction in elderly patients: A meta-analysis and systematic review.” Heart Rhythm O2 1.4 (2020): 250-258.
- Parthiban, Nirmalatiban, et al. “Remote monitoring of implantable cardioverter-defibrillators: a systematic review and meta-analysis of clinical outcomes.” Journal of the American College of Cardiology 65.24 (2015): 2591-2600.
- Lu, Lin, et al. “Wearable Health Devices in Health Care: Narrative Systematic Review.” JMIR mHealth and uHealth 8.11 (2020): e18907.
- Adibzadeh, Fatemeh, et al. “Systematic review of pre-clinical and clinical devices for magnetic resonance-guided radiofrequency hyperthermia.” International Journal of Hyperthermia 37.1 (2020): 15-27.
