
Editing And Proofreading Your Research Paper
July 5, 2021
Systemic reviews on implantable medical devices provide a quality of reporting
July 12, 2021In brief
A protocol is a document that arranges out the research strategy for a clinical study. For all elements of clinical research, it is the single most critical quality control instrument. This is especially true in multi-centre clinical research, which necessitates numerous investigators and their staff from many institutions in the study operations. Some protocol variations are insignificant, but others can affect the research’s validity. For example, a patient may be unaware of a condition that is present in its early or latent stage, or a patient may intentionally mislead a researcher into believing they will receive special treatment by participating in a study – both of these scenarios result in violations of the research protocol’s patient exclusion criteria(1).
Introduction
Clinical research is carried out following a set of guidelines (protocol) or an action plan. The protocol arranges out the procedures for carrying out the trial. It demonstrates what will be done in the study by describing each important component and how it will be carried out. It also explains the participants’ eligibility, the study duration, the medications used, and the tests performed. A chief researcher is in charge of a protocol. Members of the research team will monitor the participants’ health regularly to ensure the study’s safety and effectiveness.The journal considers protocols for existing or proposed large-scale, prospective research relevant to public health and public health management activities. Suggestions should include a thorough description of the study’s hypothesis, rationale, and methodology(2).
Table: 1 the key aims and benefits of the protocol
| Aims | Benefits |
| 1) To identify the research question and clarify its significance. 2) To gather current knowledge and discuss the work of other scholars who have undertook similar issues (Literature review). 3) To come up with a hypothesis and a set of goals. 4) To make ethical considerations more clear. 5) Propose an approach for resolving the problem and accomplishing the objectives. 6) To talk about the requirements and constraints in reaching the objectives. | The researcher can plan and review the project’s steps with this tool. Throughout the research, it acts as a guide. Estimates of time and budget are compelled.. |
- Correctly drafting the protocol will raise the possibility that the research’s conclusions are scientifically sound. Colleagues and experts should be consulted for advice and recommendations as researchers build their plans. The protocol should not be changed during the study or trials once it has begun.
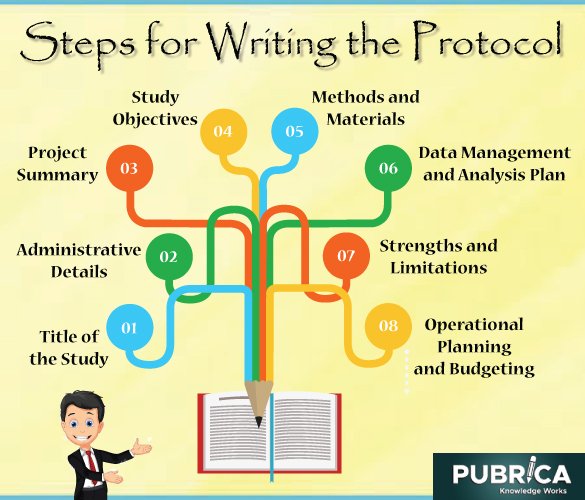
Components of a research protocol
The Clinical trials must be approved and managed by an Institutional Review Board, verifying that the risks are insignificant and that the benefits outweigh the risks. It is an independent body made up of doctors, dentists, statisticians, and community members. The committee guarantees that clinical trials are conducted ethically and that all participants’ rights are respected. The board must first approve the research and then examine it regularly (3).
Table: 2 components of the protocol
| Components |
| 1) Title of the study 2) Administrative details 3) Project summary 4) Introduction to the research topic, background (Literature review) 5) Preliminary studies 6) Study objectives and questions—statement of the difficult. 7) Study design, study population, and recruiting techniques, variables list, sample size, data collection techniques, data collection instruments, and analytic plan (analysis of data) 8) Project organization: Work plan (Timeline – proposed schedule) 9) Strengths and limitations of the study 10) Issues for ethical review and approvals |
Writing the protocol
Protocol writing enables the researcher to analyze and critically analyze published material on the research topic of interest, organize and review project processes, and serve as a guide during the study. The proposal is an essential document that allows the researcher to track the project’s progress (4).
- Title of the Study: The proposal’s title should be precise, brief, simple, and distinct. What is the purpose of the study, and who are the participants? What is the study’s setting, and, if appropriate, when will it be launched?
It should state the key goal, express the research’s principal purpose, and identify the target population. In a few words, convey as much information about the issue as possible; it’s a good idea to keep the title to 12-15 words. It should express a concise, relevant, correct, appealing, easy-to-understand, and enlightening notion about the study area and the methodologies that will be applied.
- Administrative Details: Following the title page, the following administrative details and a protocol content summary should be included:
- A list of important sections and sub-sections with page numbers can be found on the contents page.
- The signature page is signed and dated by senior members of the research team to confirm that the version in question has been accepted.
- Members of the study team’s contact information, including postal and e-mail addresses and phone numbers.
- Project Summary: The summary should be unique, concise, and cover all of the protocol’s essentials.
- Introduction (Background): The project’s background should be brief and direct in its approach to the issue. The strengths, disadvantages, and limitations of the studies
cited should be highlighted in the review. The introduction ends with an explanation of how the current study will benefit the community. The literature review services should logically lead to a declaration of the proposed project’s goals and end with the study’s goals and objectives. The review should contain the most recent papers on the subject. The study topic is chosen only after the literature evaluation has been completed and identified gaps.
- Study Objectives (Aims): The study questions/hypotheses lead to the study goals or objectives. They are reactions to the possible replies to the research topic or hypothesis being investigated and measured. Aims should be logical and cohesive, feasible, concise, and practical, considering local conditions and phrasing to satisfy the study’s purpose and be related to the specific research’s goals.
The purposes should be (SMART objective): Specific, Measurable, Achievable, Relevant and Time-based.
- Methods and Materials: It should include information on “Where,” “Who,” and “How” the study will be conducted. It covers the study’s design and the procedures and techniques employed to attain the goals. It outlines the variables and explains how they will be measured in detail. It explains the suggested data collection and processing approach. The protocol’s methodology is a key component. It guarantees that the theory will be proven or disproven. It also refers to a well-thought-out approach for achieving the goals (5).
The methods and materials are divided into several subheadings:
- Study design (case-control,cross-sectional, intervention study, RCT, etc.): A thorough explanation of why a particular expertimental design was chosen should be provided (based on proposed objectives and availability of resources). The best way to prove a causal relationship between exposure and an outcome is to conduct a randomized controlled clinical trial.
Table: 3 Suitable research design depends on the purpose of the study.
| Purpose | Study Design |
| To determine the frequency and burden of a disease | * Cross-sectional survey (Prevalence) * Cohort study (Incidence) |
| To identify the risk factors | *Cohort study * Case-Control study |
| To determine the prognosis of a disease | * Cohort study |
| To determine the efficacy/effectiveness of a new treatment | * Clinical trials * Community intervention |
| To evaluate community programs | * Evaluation |
b) Study population (Study subjects): Where will you conduct your research, and who will be the study population (why are you conducting research in this location, and why have you chosen this demographic?).
c) Sample size: Calculating the sample size is suggested for both economic and ethical reasons. The sample size computation must be described, as well as the sample’s power. The sampling strategy, such as randomization that will be utilized to acquire a representative sample for your target population, should be indicated.
d) Proposed intervention: The suggested intervention should be described in detail. All activities and acts should be documented and properly explained in the sequence in which they occurred.
e) Data collection methods, instruments used:
Data collection tools are:
- Retrospective data (medical records)
- Questionnaires
- Interviews (Structured, Semi-Structured)
- A test in the laboratory (literature or individual knowledge should be referenced if conventional test or report should be provided in details, if not recognized)
- Medical evaluations
- A description of the instruments, the tools used to collect data, and the methodologies used to test the instrument’s validity and reliability should be supplied.
7) Data Management and Analysis Plan: This part should be written with the help of a statistician’s statistical advice. The analytical plan and the statistical tests that will be utilized to verify the significance of the research question/hypothesis should be presented with suitable references. If computer applications are to be used, the software used and its version must be specified.
8) Strengths and Limitations: It is critical to state the study’s strengths and limitations, i.e., what the study can and cannot do to avoid wasting resources.
9) Ethical Considerations (Issues for Ethical Review and Approvals): It should state whether the processes to be followed comply with the Helsinki Declaration. In any case, the study should not begin until the ethical committee has approved it.The following points should be explained:
- The advantages and disadvantages for the subjects involved. The research’s physical, social, and psychological implications.
- Information to be provided to study participants, including alternative treatments and techniques.
- Information about the participants’ free and informed consent should be delivered. Justification for research, study outline, risks, confidentiality, and voluntary participation should be included in the information form. Patients should be educated of their right to withdraw from the study at any time. Confidentiality refers to how the patient’s personal information will be kept secret (Data safety).
10) Operational Planning and Budgeting (Budget Summary): Outline the budgetary requirements for the study, including staff, transportation, instruments, laboratory testing, and drug costs. An annexure with a budget estimate is required. The budget includes all costs, including people, consumables, equipment, supplies, communication, and funds for patients and data processing. Justification is required for each item.
11) Reference System: The standard way of acknowledging information drawn from the work of other academics is referencing. Readers will be able to follow up on any references of interest if they are properly cited. Plagiarism is the act of claiming and gaining someone else’s ideas without their permission, and it is a crime.
Plagiarism is defined as failing to acknowledge the contribution of other team members in a group assignment or failing to cite an idea uncovered in your research. As a result, reference is a critical component of the research protocol.The Vancouver system and the Harvard system are the two most often used citation methods in clinical writing. The referencing system chosen is determined by the funding organizations to which the study protocol is submitted. These typically specify their preferred referencing system, which should be properly followed. The Vancouver style is the most widely used in dental literature review (6).
Conclusion
The drafting of a protocol, which results in a brief but comprehensive document that summarises the project, is the most difficult stage of executing a research project. When a proposal is straightforward, free of typographical errors, accurate, and easy to understand, it is successful. To conduct an acceptable study and receive truthful results, it is critical to understand the stages involved in designing a research protocol. The extra time invested in writing a strong protocol will save failures later on and assist data analysis. If the protocol is not properly written and followed, it is doubtful that the project will deliver the results you desire. Your chances of selling your proposal to awarding agency evaluators will be reduced(7).
References
- Alvariza, Anette, et al. “Increasing preparedness for caregiving and death in family caregivers of patients with severe illness who are cared for at home–study protocol for a web-based intervention.” BMC palliative care 19.1 (2020): 1-8.
- Lühnen, Julia, et al. “Efficacy of a training programme to support the application of the g uideline evidence-based health information: study protocol of a randomized controlled trial.” Trials 21 (2020): 1-14.
- Carøe, Christian, and Kristine Bohmann. “Tagsteady: a metabarcoding library preparation protocol to avoid false assignment of sequences to samples.” Molecular Ecology Resources 20.6 (2020): 1620-1631.
- Moullin, Joanna C., et al. “Systematic review of the exploration, preparation, implementation, sustainment (EPIS) framework.” Implementation Science 14.1 (2019): 1-16.
- Mousavi, SeyyedMeysam, AmirhosseinTakian, and Mahmood Tara. “Design and validity of a questionnaire to assess national eHealth architecture (NEHA): a study protocol.” BMJ open 8.12 (2018): e022885.
- Al-Jundi A, Sakka S. Protocol Writing in Clinical Research [published correction appears in J ClinDiagn Res. 2016 Dec;10 (12 ):ZZ03]. J ClinDiagn Res. 2016;10(11):ZE10-ZE13. doi:10.7860/JCDR/2016/21426.8865
- Christopher, Michael, Sarah Bowen, and Katie Witkiewitz. “Mindfulness-based resilience training for aggression, stress and health in law enforcement officers: study protocol for a multisite, randomized, single-blind clinical feasibility trial.” Trials 21.1 (2020): 1-12.



