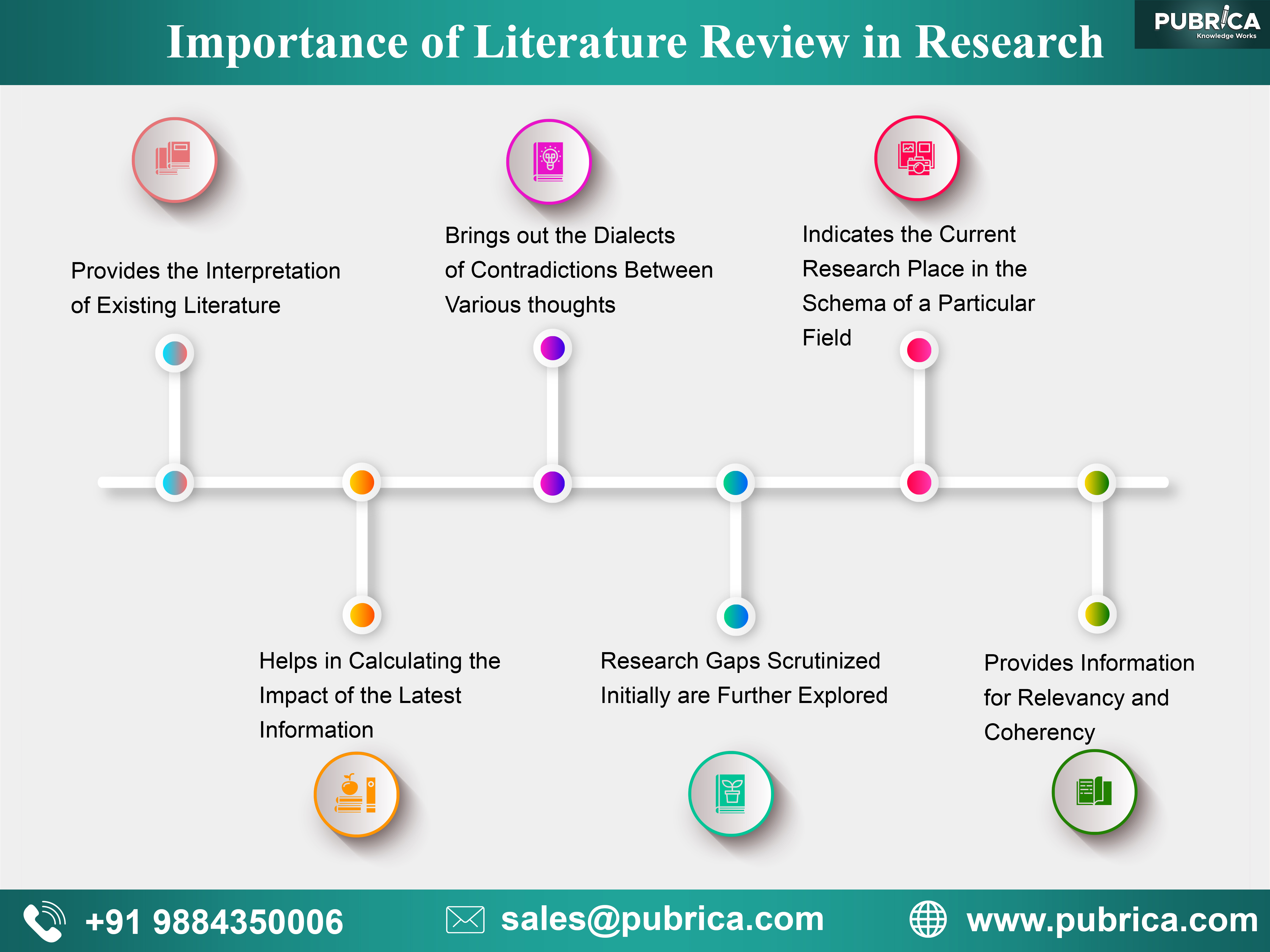
Why is it important to do a literature review in research?
August 8, 2019
On Biostatistics and Clinical Trials
August 21, 2019Statistics is the grammar of science – Karl Pearson
Biostatistics role and importance in clinical research started way back in the 17th century and continues to grow stronger. After helping in the work of scientific greats like Charles Darwin, Karl Parson, and others, it is now helping the budding researchers in clinical research. Biostatistics also helps in presenting the scientific manuscript with relatively sophisticated statistical analyses of a complex set of medical data in renowned scientific journals.
Biostatics in Evidence-Based Clinical Practice and Drug Development:
The biostatistical analysis is key to conduct new clinical research and one of the foundations of evidence-based clinical practice. It evaluates and applies prior research findings precisely for the new researches. With less than 10 per cent of the new compounds reaching the market, the need for advanced biostatistics is increasing every day. It is because it shortens timelines, reduces costs and risks by improving the submission quality. State of the art statistical methods now plays a key role in all the steps of the drug development process.
Biostatics Application in various fields of Clinical Research:
Clinical researches utilize biostatistics methods to provide formal accounting for sources of variability in patients’ response to treatment. Also, it allows researchers to draw reasonable and precise inferences from the gathered information to make outstanding decisions in times of uncertainty. It enables the collection, analyzing, presenting and interpreting data to find application in various field like
- Epidemiology
- Clinical trials
- Population genetics
- Systems biology
Biostatistics help in Clinical Researches right from its start for:
- Designing
- Conducting
- Analyzing
- Reporting
- Minimizing biases
- Confounding factors
- Measuring random errors
- Understanding the research
- Make suggestions on hypothesis testing & analysis
- Calculating the sample size
- Determine the power of the study
- Ensure continuity throughout the research
- Assess the statistical significance of the results
- Efficacy & safety of the drug
- Line of treatment
- Therapy
Biostatistical reasoning is characterized by:
- Establishes an objective framework to conduct investigations
- Places both data and theory on an equal scientific footing
- Data production is designed through experimentation
- Quantifies the influence of chance
- Estimates systematic and random effects
- Combines theory and data by way of formal methods
Biostatistics prevent fraud in Clinical Research:
In recent times there have been many reports in the media glare about the frauds in the clinical trials. Biostatistics also prevents fraud or unintentional errors during clinical research. The fraud happens due to data fabrication or making up data values or falsification, which is changing data values. As per the reported cases, it involves cheating on the inclusion criteria for the ineligible persons to enter the trial. Also, by fabricating the data, the necessary data for such wrong inclusions are made not missing.
Biostatics have come on leaps and bounds with P-value:
With the continuous evolution of the biostatistical methods for the past few centuries, now they can help in experimental design with a prospective approach. The p-value which came into existence only 80 years ago has made the scope and use of biostatistics leap in bounds. P-value summarizes whether the observed data could have happened by chance and is widely used in clinical research involving:
- Investigating proposed medical treatments
- Assessing the relative benefits of competing therapies
- Establishing optimal treatment combinations
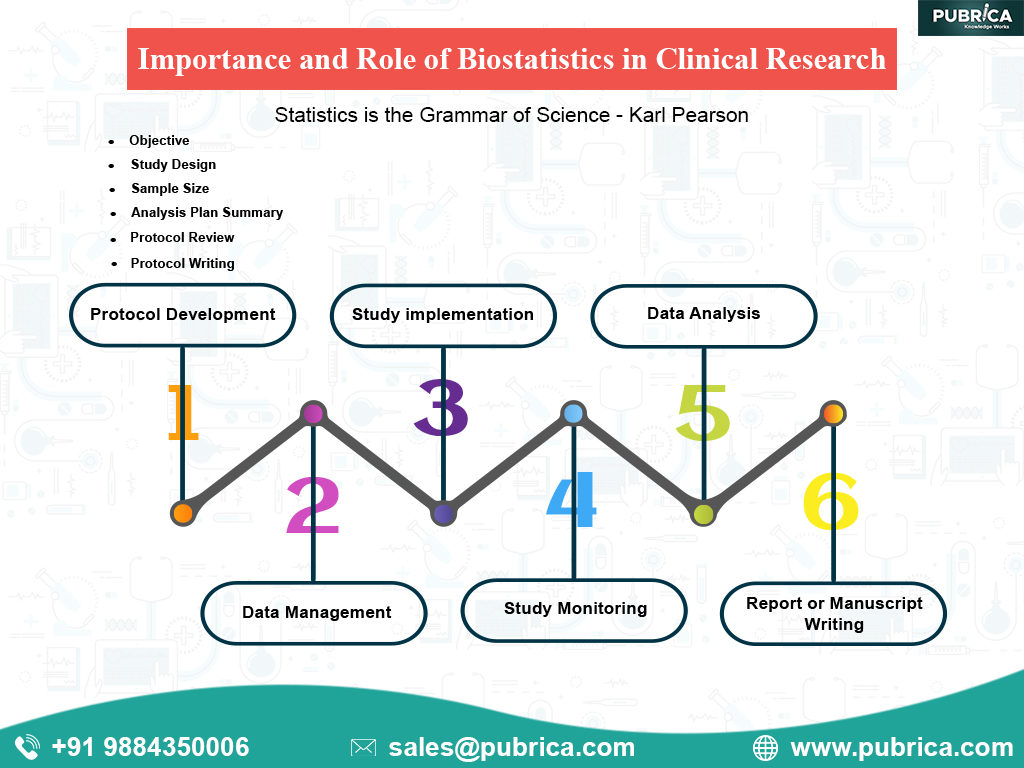
Roles and Importance of Biostatistics in Clinical Research:
1.Protocol Development
In the protocol development in clinical researches, the following are the roles and responsibilities of the biostatistician
- Objectives:
Based on the objective of the scientific manuscript, biostatisticians have to provide a clear specification of the hypothesis to be tested. In other words, they have to provide the parameters to be tested. Also, they are responsible for selecting and defining endpoints in clinical research.
- Study Design:
The study design of the biostatistician should provide the data needed to answer the objectives like:
- Defining procedures to minimize selection bias
- In the case of RCT or Randomized Control Trial, define randomization procedures like sequence generation and allocation concealment and the length to follow up and frequency of contacts.
- Sample size:
- Justifying the primary endpoint in terms of power or precision
- Methods used to calculate the sample size should be consistent with the primary method of data analysis and also appropriate for the design
- Assumptions should be supported with proper historical data.
- Justification in terms of feasibility
- Analysis plan summary:
- The purpose of analyzing plan summary is to assure objectives to be achieved and to justify design and data collection byways like
- To provide the statistical methodology for the assessment of the primary objectives like testing procedures & statistical hypothesis
- As per the role of DSMB or the Data Safety Monitoring Board to discuss statistical methods to be used in planned interim analyses
- Protocol Review:
It is pertinent for the LSB or the lead study biostatistician to review the full protocol for checking the following
- Clarity
- Completeness
- Consistency
- Data quality issues
- Feasibility
- Protocol writing:
To write randomization procedures, sample size, and analysis plan with the inputs from objectives, endpoints study design, and allocation concealment.
2. Data Management:
- CRF management with content and design
- Dataset specification with annotation of CRFs and record layout
- Validation with error checking specification and test data
3. Study Implementation
It involves sampling selection and implementation of randomizes procedures
4. Study Monitoring
It involves monitoring of quality and for safety and efficacy
5. Data Analysis:
- A detailed analysis plan writing with all hypotheses to be tested along with the hierarchy of analysis
- It helps to prepare for reporting, manuscript writing, along with the validity and creditability of results
6. Reports or Manuscript writing:
- Method section with statistical methodology and description of data with endpoints and design
- Result section includes data presented in the form of a graph, tables, and others
- Discussion section with the appropriate interpretation of the results
Biostatistics has become crucial for the modern-day clinical researches to understand them better and for various other uses.
References:
http://www.icssc.org/Presentations/Aregentina%20Presentations/11%20The%20role%20of%20BIOS.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4900305/
https://newonlinecourses.science.psu.edu/stat509/node/2/
Tags:
journal Publishing services | Scientific Editing Services | Medical Writing Services | scientific research writing service | Scientific Medical communication service | Biostatistical Programming
Related Topics:
Scientific Research Paper Writing
