
How Randomized Controlled Trials are Used in Meta-Analysis
October 26, 2023
What are the key points to reflect while considering the case report series in BMJ?
November 16, 2023How Randomized Controlled Trials are Used in Meta-Analysis
Randomized controlled trials—for efficacy research
Randomized controlled trials (RCTs) are prospective studies that assess the efficacy of a novel intervention or treatment. Although no research is likely to show causation, randomization eliminates bias and provides a rigorous technique for examining cause-effect correlations between an intervention and result. This is because the process of randomization balances participant characteristics (both observable and unobserved) between groups, allowing any variations in result to be attributed to the research intervention. This is not achievable with any other research design.RCTs are Used in Meta-Analysis
Randomized Controlled Trials (RCTs) are a commonly used research design in medical and scientific studies to assess the effectiveness of interventions or treatments. Meta-analysis, on the other hand, is a statistical technique used to combine and analyze the results of multiple studies on a particular topic to draw more robust conclusions.
- To know more about meta-analysis services, check our study guide on How to do a meta-analysis for a manuscript.
Here’s how RCTs are used in the context of meta-analysis:
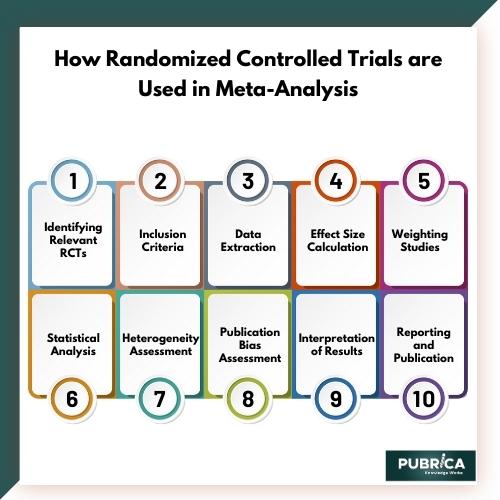
- Identifying Relevant RCTs: The first step in a meta-analysis is to identify all relevant RCTs that have been conducted on a specific topic. This is typically done through a systematic literature review, where researchers search various databases and sources to find all available RCTs.
- Inclusion Criteria: Researchers establish specific inclusion and exclusion criteria to determine which RCTs are eligible for inclusion in the meta-analysis. Common criteria might include the type of intervention, the patient population, the outcome measures, and the study design.
- Data Extraction: For each eligible RCT, relevant data is extracted. This includes information about the study design, sample size, treatment group, control group, and the results in terms of the outcome of interest. This data collection is typically recorded in a standardized format.
- Effect Size Calculation: One of the key steps in meta-analysis is calculating the effect size for each RCT. The effect size is a measure of the magnitude of the treatment effect, and it allows for the comparison of results across different studies. Common effect size measures include odds ratios, risk ratios, or mean differences, depending on the type of data and outcome being studied.
- Weighting Studies: In a meta-analysis, not all studies are given equal importance. Larger studies or those with a lower risk of bias may be given more weight in the analysis. This helps ensure that the results of higher-quality studies have a greater influence on the overall findings.
- Statistical Analysis: Meta-analysis employs statistical techniques to combine the effect sizes from individual RCTs. A common method is to use a weighted average of the effect sizes, where the weights are determined by the study’s sample size and quality.
- Heterogeneity Assessment: Researchers assess the heterogeneity of the included RCTs to determine how much variability exists between the studies. This is important because high heterogeneity may impact the reliability of the meta-analysis results.
- Publication Bias Assessment: Publication bias occurs when studies with positive results are more likely to be published, leading to an overestimation of the treatment effect. Meta-analysts often assess for publication bias and attempt to correct it.
- Interpretation of Results: The meta-analysis results are interpreted to provide an overall estimate of the treatment effect, along with a measure of uncertainty (e.g., a confidence interval). This synthesis of evidence from multiple RCTs allows for a more comprehensive and reliable assessment of the intervention’s efficacy.
- Reporting and Publication: The findings of the meta-analysis are typically reported in a scientific paper or a report, and they may be subject to peer review and publication in academic journals.
Meta-analysis of RCTs is a powerful tool for summarizing and synthesizing the evidence from multiple studies, providing a more comprehensive understanding of the effectiveness and safety of a particular intervention or treatment. It helps decision-makers, clinicians, and policymakers make more informed choices based on a broader and more reliable evidence base.
- Check Examples to get an understanding of our adaptability across meta-analysis topics and subject domains.
Conclusion
In conclusion, Randomized Controlled Trials (RCTs) play a pivotal role in the process of meta-analysis, which combines and analyzes the results of multiple studies to derive robust conclusions. By systematically identifying, extracting, and analyzing data from relevant RCTs, researchers can gauge the effectiveness of interventions with greater precision. The use of effect size calculation, weighting of studies, and statistical analysis allows for a comprehensive synthesis of evidence, minimizing bias and offering a more dependable overview of an intervention’s impact. This evidence-based approach empowers decision-makers, clinicians, and policymakers to make informed choices, enhancing the quality of healthcare and scientific decision-making.About Pubrica
Pubrica has extensive experience in conducting meta-analysis, a quantitative, formal, epidemiological study design used to systematically assess the results of previous research to derive conclusions about that body of research. Pubrica’s team of researchers and authors develop Scientific and medical research papers that can act as an indispensable tool to the practitioner/authors. Pubrica medical writers help you to write and edit the introduction by introducing the reader to the shortcomings or empty spaces in the identified research field. Our experts are aware of the structure that follows the broad topic, the problem, and the background and advance to a narrow topic to state the hypothesis.References
- Hariton E, Locascio JJ. Randomized controlled trials – the gold standard for effectiveness research: Study design: randomized controlled trials. BJOG. 2018 Dec;125(13):1716. doi: 10.1111/1471-0528.15199. Epub 2018 Jun 19. PMID: 29916205; PMCID: PMC6235704.
- Gegenfurtner, Andreas, and Christian Ebner. “Webinars in higher education and professional training: A meta-analysis and systematic review of randomized controlled trials.” Educational Research Review 28 (2019): 100293.
Tags
- Pubrica Meta-Analysis Solutions for Product Development and Refinement
- Meta-Analysis Services for Case-Control Studies
- Meta-Analysis Solutions for Drug Discovery
- Multiple Treatment Meta-Analysis Research Solutions

14 years of expertise in clinical research with a doctoral distinction in life science.


