
What are the limitations of the QUADAS-2 tool?
April 6, 2023
How to Identify Dependent, independent & Moderators from a research question
April 12, 2023In brief
QUADAS- 2 was used by three reviewers to independently assess the quality of 30 research. They calculated the percentage of agreement between each reviewer and the overall consensus rating. This was done for all QUADAS- 2 items as a group as well as for each individual item. Twenty reviewers who have utilized QUADAS- 2 in their evaluations completed a brief, structured questionnaire about their QUADAS- 2 experience.
Introduction
The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool is used to assess the quality of primary studies included in a systematic review of diagnostic accuracy studies. Here are the steps to use the QUADAS-2 tool:
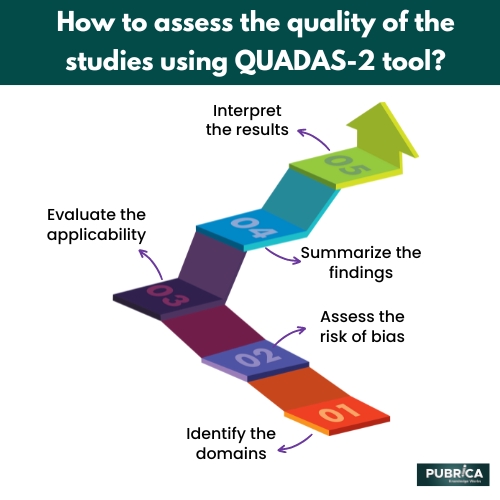
- Identify the domains: The QUADAS-2 tool consists of four domains: index test, patient selection, reference standard, and flow and timing. Reviewers should consider each domain for each study included in the systematic review.
- Assess the risk of bias: Reviewers should assess the risk of bias for each domain using signalling questions specific to each domain. The signalling questions are used to determine the risk of bias for the individual domains as “low,” “high,” or “unclear.”
- Evaluate the applicability: Reviewers should evaluate the applicability of the studies by considering whether the study population, index test, and reference standard are similar to the population, test, and standard of interest in the systematic review.
- Summarize the findings: Reviewers should summarize the findings of the applicability assessments and risk of bias for each study and domain.
- Interpret the results: Reviewers should interpret the results of the QUADAS-2 assessment in the context of the systematic review. This may involve identifying studies with a low risk of bias and high applicability and excluding studies with a high risk of bias or unclear applicability.
The QUADAS-2 tool can be used to assess the quality of individual studies included in a systematic review of diagnostic accuracy studies and to identify sources of heterogeneity between studies. The tool has been validated and is widely used in systematic reviews of diagnostic accuracy studies.
Conclusion
QUADAS- 2 had generally positive scores from reviewers for coverage, ease of use, clarity of instructions, and validity, particularly for coverage, which was scored as good or very good by all reviewers, and ease of use, which was rated as at least average by all reviewers (1).
About Pubrica
Pubrica’s team of researchers and writers generates scientific and medical research articles that might be significant resources for practitioners and authors. Pubrica medical writers aid you in creating and rewriting the introduction by informing the reader of the gaps in the selected research field. Our experts are aware of the sequence in which the broad topic, issue, and background are followed by the particular subject in which the hypothesis is given.
References
Whiting, P.F., Weswood, M.E., Rutjes, A.W. et al. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 6, 9 (2006)
