
Selecting materials for medical device industry
May 1, 2021
The Role of Packaging Design In Drug Development
May 6, 2021Introduction:
Crude materials in pediatric formulations, for example, excipient, food ingredients, and active pharmaceutical ingredients (APIs) have gotten significant consideration from administrative offices worldwide lately because of wellbeing concerns. Not all excipient and food ingredients are “inactive” and have been appeared to meddle with the development and improvement measures in the pediatric populace. In spite of the fact that crude materials used in drug items require broad security testing preceding their consideration in the details, there are not many excipient that have gone through randomized clinical trials (RCT) in the pediatric subpopulation.
Excipient
Drug excipient expected for fuse into measurement dosage is endorsed ingredients that are considered “dormant” and by and large, perceived as protected (GRAS) for human utilization. Excipient make up the majority of any medication item and are incorporated to confer security; guarantee exactness and accuracy, homogenous mixing, cover the harsh taste, improve flow ability, add mass thickness, and control the arrival of API subsequently improving patient consistency, bioavailability, viability, and decrease poisonousness of the API. In spite of the fact that excipient are considered “idle” and can be absolved from posting on certain medication items, they are needed by the FDC Act to be recorded on ophthalmic, skin, and parenteral items. Subsequently, excipient are exposed to the thorough present moment and long haul toxicological examinations preceding their consideration in drug items for grown-up populace yet are not tried in the pediatric subpopulation.
Classification of Excipient
Excipient can be grouped relying upon the origin of source like plant, creature, mineral, and engineered based, the useful job they play in the definition like fasteners, diluents, disintegrants, fillers or building specialists, glidants, oils, shading specialists, additives, sweeteners, surfactants, solvents, covering specialists, and synthetic substituents present in the excipient like alcohols, acids, esters, starches, glycerides, halogenated subordinates, mercury salts, sulfites, and so on.
Active Pharmaceutical Ingredient
Active Pharmaceutical Ingredient (API) is the organically active part of a medication item (tablet, case, and cream, injectable) that delivers the planned impacts. APIs discover applications in top-notch sedates that treat sicknesses relating to oncology, cardiology, CNS and nervous system science, muscular, pulmonology, gastroenterology, nephrology, ophthalmology, and endocrinology. APIs can make a more feasible medical care framework by presenting more creative items.
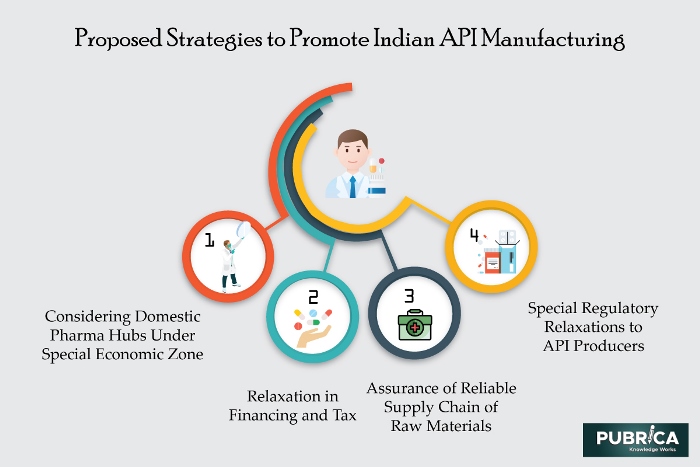
Figure: Future strategies to make self-resilient India in API manufacturing
Aside from APIs, a medication contains synthetically idle parts named ‘excipient’, which convey the impact of APIs on the human body framework. An API producer initially builds up the substance compound in a lab, after which the creation division fabricates a mass measure of APIs utilizing enormous size reactors. At last, these are checked for immaculateness before offering it to the medication creators. The nature of an API is perhaps the main element considering the fitting adequacy of the medication. Besides, the dynamic drug fixings (APIs) market in our nation is estimated to achieve an income of $6 billion before the finish of 2020.
Conclusions
Excipient serves numerous functions in a plan by improving item conveyance as an assimilation enhancer and improving an API’s flow properties during assembling measure. Hence, there are not many medication items that can be fabricated without an excipient. Excipient fused in pediatric details requires wellbeing assessment in a specific subset of the pediatric populace because of the changeability in ADME profile among the subpopulation. RCT to assess the wellbeing of excipient in the pediatric populace is restricted by the accessibility of pediatric patients and blood tests and difficulty in extrapolating the outcomes to the pediatric subpopulation using clinical research services. It is exceptionally improbable that all excipient would be exposed to RCT in the pediatric populace to such an extent that the suggested day-by-day admission could not be resolved, nor there a rundown of “chose excipient” that could be solely utilized in the pediatric populace. Thusly, it would be to the greatest advantage of the scientific research local area to assess the wellbeing profile of the excipient remembered for the medication items throughout the medication improvement measure in the pediatric populace.
References:
- Katsura-chemical.co.jp [Internet]. Japan: What is an API; c2020. Available from: https://www.katsura-chemical.co.jp/en/drugs.
- Zhang, H., Hua, D., Huang, C., Samal, S. K., Xiong, R., Sauvage, F., … & De Smedt, S. C. (2020). Materials and technologies to combat counterfeiting of pharmaceuticals: current and future problem tackling. Advanced Materials, 32(11), 1905486.
- Wang, D., Cheow, W. S., Amalina, N., Faiezin, M., & Hadinoto, K. (2021). Selecting optimal pharmaceutical excipient formulation from life cycle assessment perspectives: A case study on ibuprofen tablet formulations. Journal of Cleaner Production, 292, 126074.
- ExcipientQualificationGuide.pdf
- Biswas D, Sultana P. Policing during the time of corona: The Indian context. Policing 2020;0:1-8.



