
Epidemiology designs for clinical trials
April 8, 2021
How EBP enable healthcare professionals to provide informed decision
April 15, 2021Introduction to Health Economics
The development of significant expense medicines has sped up the speed of conversations about elective installment models that would at the same time empower fast persistent access while guaranteeing manageable medical care spending. Nonetheless, restricted exact research exists on the greatness of the issue and the normal exhibition of such payment models. A critical hindrance to directing clinical trials is their significant expense, which is driven principally and assets needed to actuate trials and arrive at accumulation targets. The significant expense of running trials considerably affects their drawn-out practicality and the kind of clinical examination attempted.
Applications of Health Economics
Healthcare Economics is an applied field of study that considers the methodical and thorough assessment of the issues looked at in advancing wellbeing for all. By applying financial speculations of the purchaser, maker, and social decision, wellbeing financial aspects expect to comprehend the conduct of people, medical services suppliers, public and private associations, and governments in dynamic.
Healthcare financial matters are utilized to advance sound ways of life and positive healthcare results through the investigation of medical care suppliers, medical clinics, and facilities, overseen care, and general wellbeing advancement exercises. The MHS in Global Health Economics degree program in the Department of International Health at the Johns Hopkins Bloomberg School of Public Health utilizes wellbeing monetary administrators to address worldwide issues like movement, uprooted people, environmental change, antibody access, wounds, corpulence, and pandemics.
Healthcare market analysts apply the hypotheses of creation, productivity, aberrations, rivalry, and guideline to all the more likely illuminate general society and private area on the most proficient, practical, and impartial strategy. Such exploration can incorporate the financial assessment of new innovations, just as the investigation of proper costs, hostile to confide in arrangement, ideal public and private venture, and key conduct.
Recent trends in Health economics
Another ISPOR* report depicts 2018’s main 10 trends in the utilizations of Health Economics and Outcomes Research (HEOR).
Summed up from the ISPOR report:
1. Medication Pricing and Spending. Discussions on the best way to oversee drug costs are getting more typical, with a specific spotlight on guaranteeing that medication costs mirror the estimation of treatment.
2. Inventive and Curative Therapies. As medication revelation and medical services push toward more customized clinical therapy, novel treatments will keep on being created. This is probably going to convey more prominent positive wellbeing sway, yet added spending pressures. The job of HEOR will be imperative to control choices on whether and how to subsidize such treatments.
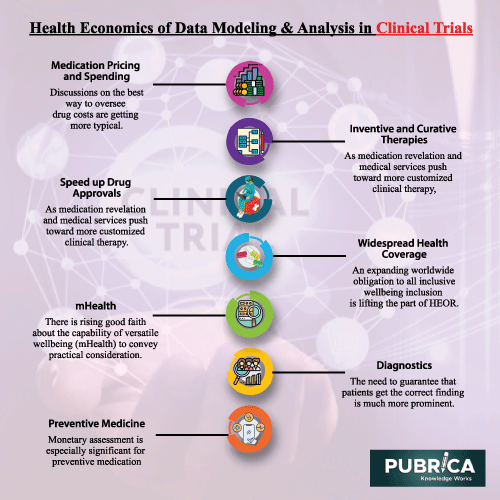
3. Speed up Drug Approvals. Administrative endeavors keep on speeding the endorsement of new medications, particularly in territories of neglected clinical need. Quicker market section may convey significant medical advantage, yet this should be compromised against any decrease in security.
4. Widespread Health Coverage. An expanding worldwide obligation to all inclusive wellbeing inclusion is lifting the part of HEOR. While such frameworks have been the norm in Europe for quite a long time, the World Health Organization’s attention on general medical services may raise its profile and focus on it in different settings.
5. mHealth. There is rising good faith about the capability of versatile wellbeing (mHealth) to convey practical consideration. Similarly as with any new innovation, nonetheless, assessment can assist with guaranteeing that mHealth accomplishes its latent capacity.
6. Diagnostics. As treatments become further developed and all the more exorbitant, the need to guarantee that patients get the correct finding is much more prominent.
7. Preventive Medicine. Monetary assessment is especially significant for preventive medication, which holds incredible potential to improve medical services, especially in low-and center pay nations.
Healthcare economics in Clinical Trials
Thirty years prior there were restricted choices for specialists settling on treatment decisions and patients got in line. Any qualities that added to the dynamic cycle were verifiable and dictated by the doctor. Nonetheless, against a foundation of restricted healthcare analytics services assets, an engaged customer, and an expanding cluster of intercession alternatives, there is a requirement for choices to be taken all the more straightforwardly and reasonably.
Versatile plans offer an adaptable methodology, permitting changes to a preliminary dependent on assessments of the information as it advances. Clinical trials are turning into a well-known decision, as the reasonable utilization of limited exploration financial plans and precise dynamic need for medical services suppliers all throughout the planet. The techniques for wellbeing financial matters, which plan to expand the well-being acquired for cash spent, could be fused into the plan and investigation of clinical preliminaries to make them more effective. The utilization of healthcare financial aspects in the plan and investigation of clinical preliminaries can possibly build the proficiency of healthcare innovation appraisals around the world.
The significance of the financial model is that it gives helpful bits of knowledge into how medical care can be coordinated and financed and gives a structure to address a wide scope of issues in an express and steady way. Authoritative changes, for example, the improvement of the National Institute for Clinical Excellence and the devolution of dynamic to essential consideration associations have prompted an expanding interest in the subject and its effect on medical care association and dynamic.
Conclusion
There is a requirement for more economic evaluations of the advantages of clinical research, for example, healthcare framework use (or shirking) and healthcare results in urban areas and healthcare specialists with organizations that lead clinical studies, to exhibit the moderateness of clinical trials, notwithstanding their significant expense. Future research is expected to all the more likely comprehend boundaries to the consideration of monetary endpoints just as how much fusing medical services asset use gathered during clinical preliminaries into early financial demonstrating may diminish payer worries about model straightforwardness and inclination.
References:
- Colene Bentley, Sonya Cressman, Kim van der Hoek, Karen Arts, Janet Dancey, Stuart Peacock, 2019 Jan 10, Conducting clinical trials-costs, impacts, and the value of clinical trials networks: A scoping review
- Howard, Brandon; Health, JH Bloomberg School of Public. “What Is Health Economics?” Johns Hopkins Bloomberg School of Public Health. Retrieved 25 February 2020.
- National Health Policy. (2019, January 7). Ayushman Bharat. Retrieved from National Health Policy
- FDA. Adaptive designs for clinical trials of drugs and biologics: Available: guidance for industry. Washington; 2019
- Fogel D. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemp Clin Trials Commun. 2018;11:156–64.
- Thorlund K, Haggstrom J, Park J, Mills E. Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ. 2018;360:k698.
- Cerqueira FP, Jesus AMC, Cotrim MD. Adaptive design: a review of the technical, statistical, and regulatory aspects of implementation in a clinical trial. Ther Innov Regul Sci. 2020;54:246–58.
