
Experimental Study Design Types Methods and Advantages
March 3, 2022
How to write a Manuscript: Components and Structure of Manuscript writing
March 10, 2022Medical case report writing summarizes significant scientific findings that were overlooked or undetected in clinical trials. This can include an uncommon or unusual clinical condition, a previously unreported or undiagnosed disease, unique medication side effects or treatment response, and novel imaging modalities or diagnostic tests used to aid in disease identification. In general, a case report should be concise and focused, with the abstract, introduction, case description, and discussion serving as the primary components.
Case reports that are preciously prepared and examined with precaution play an essential part in the growth of medical case report writing knowledge and design and approach.
Five potential contributions to the defence of case report publication include:
- A new disease has been identified and described.
- Rare manifestations of a known disease are recognized.
- The discovery of a disease’s mechanisms
- Detection of harmful or favourable drug side effects (and other treatments)
- Medical auditing and education
Case Report Guidelines
CARE (Case Report) guidelines for systematic reporting of case reports were produced utilizing a consensus-based procedure. To write a solid case report, you must explain why and how a particular finding is noteworthy in the existing body of knowledge. Clinical case report writing practitioners can meet the need for transparency, clarity, and completeness of case reports by following the 13-item checklist. As a result, CARE guidelines have been endorsed by some biomedical journals. The checklist’s main components are the title, keywords, abstract, introduction, patient information, clinical case report findings, timeline, diagnostic assessment, therapeutic interventions, follow-up and outcomes, discussion, patient perspective, and informed consent.
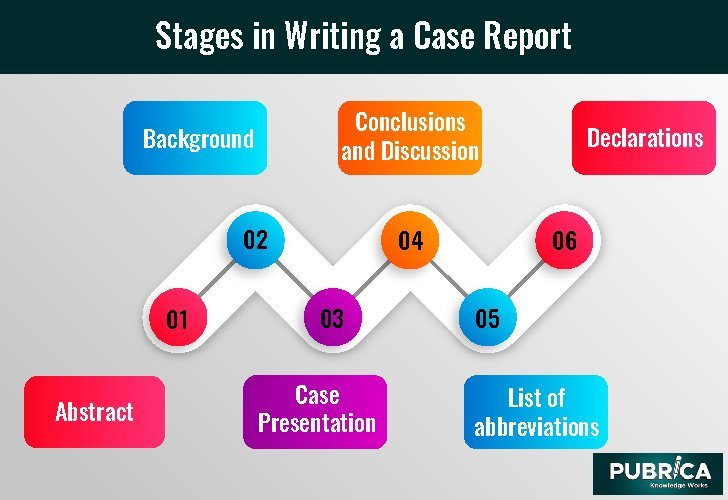
Stages in Writing a Case Report:
Introduction
Content should be concise, with no more than three paragraphs:
- Explain the case report.
- Include background details as well as relevant definitions.
- Describe the patient’s situation.
Patient’s Case Presentation
Ascertain that the patient’s case presentation has sufficient information for the reader to determine the case’s validity:
- Patient demographics (age, gender, height, weight, and so on)—avoid using patient identifiers (date of birth, initials).
- The patient’s grievances.
- Before admission, the patient’s current ailment and medical case report/family/social/medication history are.
- The name of each drug, as well as its strength, dose form, route, and administration dates.
- Completed diagnostic procedures that are relevant to and support the case, as well as their key findings.
- Histopathology, roentgenograms, electrocardiograms, skin symptoms, or anatomy photographs
- Consent from the patient and conformity to the institution’s policies.
Abstract
The abstract should be no more than 350 words long. Abbreviations should be used sparingly, and references should not be cited in the abstract. The following sections must be included in the abstract:
Background: Why should the case be reported, and what makes it unique
Case presentation: A brief explanation of the patient’s clinical and demographic details, the diagnosis, interventions, and outcomes are included.
Conclusions: A brief review of the case report’s clinical study report significance or prospective implications.
Keywords
Three to ten keywords that represent the article’s core material.
Background
The context of the case report or study, its goals, and an overview of the current literature should be explained in the Background section.
Case Presentation
This part should include a description of the patient’s relevant demographic information, medical case report history, symptoms and signs, therapy or intervention, outcomes, and any other pertinent information.
Conclusions and Discussion
This section should go over the relevant existing literature and express the main conclusions, along with an explanation of their relevance or significance to the field.
List of abbreviations
If abbreviations are used in the text, they should be defined, and a list of abbreviations should be provided.
Declarations
Under the category ‘Declarations,’ all papers must include the following sections:
- Consent to participate and ethics approval
- Consent to be published.
- Data and supplies are readily available.
- There are competing interests.
- Funding.
- Contributions of the authors
- Acknowledgements.
- Information about the authors (optional).
Details on the information to be included in these sections can be found below.
Please include the headline and add Not applicable for any sections that are not relevant to your manuscript.
About Pubrica
Pubrica has a lot of experience writing a detailed clinical case report that includes a patient’s symptoms, signs, diagnosis, therapy, and follow-up. Case reports are a significant, timely, and relevant study design in advancing medical case report writing and scientific knowledge, particularly in uncommon diseases. Case studies demonstrate the decision-making process, allowing other doctors to apply lateral thinking to their problems. Case studies should serve as informative examples for those who may face similar issues.
