Rob 2: A revised Cochrane risk-of-bias tool for randomized trials
Rob 2 (Revised Cochrane Risk-of-Bias 2) stands as a pivotal advancement in assessing the methodological quality and bias of randomized trials. Developed by the Cochrane Database Collaboration, this tool addresses the limitations of its predecessor, Rob 1, offering a more comprehensive framework for evaluating trial design, conduct, and reporting.
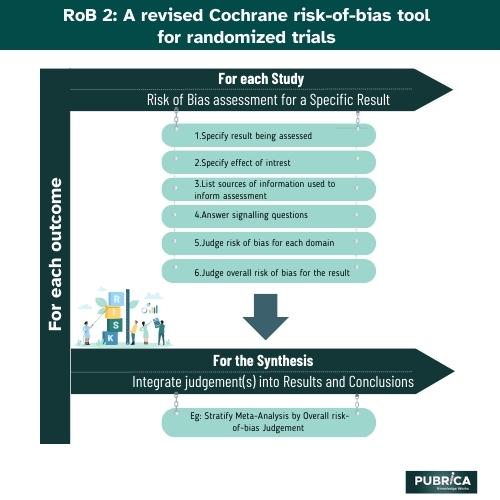
Rob 2 encompasses five domains, each representing distinct sources of bias:
- randomization process,
- deviations from intended interventions,
- missing outcome data,
- measurement of outcomes, and
- selection of the reported result.
The medical data collection tool provides signalling questions to guide reviewers in assigning bias judgments for each domain, categorizing the Cochrane risk of bias tool as low, some concerns, or high. This nuanced approach allows a more accurate medical data systems collection assessment of individual trial risks.
The data collection tool’s key innovation lies in its “Risk-of-Bias Summary” and “Risk-of-Bias Graph.” These visual aids concisely communicate the overall risk of bias assessment across domains, offering an easily interpretable snapshot of trial quality. This facilitates transparent reporting, promoting readers’ deeper understanding of trial limitations.
Rob 2 empowers researchers to make informed decisions about the validity of trial findings and the potential impact of bias. Its standardized approach enhances consistency among systematic reviews, fostering credibility in evidence-based healthcare. However, implementation challenges and user training are considerations, as Rob 2 demands a thorough grasp of trial methodology.
In conclusion, Rob 2 emerges as a refined and indispensable tool in evidence synthesis. Addressing its predecessor’s shortcomings and providing a more granular evaluation of bias contributes significantly to the rigour and reliability of systematic reviews, enabling better-informed healthcare decisions.
References
Sterne, Jonathan AC, et al. “RoB 2: a revised tool for assessing the risk of bias in randomized trials.” bmj 366 (2019).
