
Use of ICMJE URM for ethical guidance
April 1, 2021
Health economics in clinical trials
April 8, 2021Introduction:
Choosing the best accessible preventive and therapeutic measures to evade disability and passing is a significant objective as far as wellbeing might be concerned specialists. To accomplish this objective, we need to perform considers that decide the estimation of these actions. Epidemiology research alludes to examining ailment, infections, and causative reasons in population; epidemiology fills in as the highest quality level of population health appraisal. Accessory, cross-sectional, and case-control considers are out and out demonstrated as observational investigations. Consistently these studies are the fundamental commonsense strategy for thinking about various issues. A clinical preliminary’s vital place is exploring the distinction of the patient gatherings caused exclusively by the treatment strategies that are applied.
Clinical trial study design
Our goal in clinical research is to design a study that will enable us to draw a true and significant scientific conclusion using statistical methods that can be applied in a “real world” environment. Before deciding on a research design, one must first define the study’s goals and objectives, as well as choose a target population that is representative of the population being studied. The findings of a research study can either enhance health care or cause damage to patients inadvertently. As a result, a well-designed clinical research study with a strong foundation of comprehensive methodology and adherence to ethical standards is required.
- From an epidemiological perspective, there are two most important types of clinical study designs, Observational study design and Experimental study design.
- Observational studies are hypothesis‐generating studies, and they can be again divided into descriptive studies and analytic studies.
- Descriptive observational studies describe the exposure and/or the outcome, while analytic observational studies assess the relationship between the exposure and the outcome.
- Hypothesis research studies, on the other hand, are experimental studies. It entails a procedure for determining whether there is a connection between the exposure and the outcome.
- Each study design is distinct, and so it would be critical to choose a design that would most properly answer the question and provide the most useful information. We will be reviewing each study design in detail.
Observational Study Designs
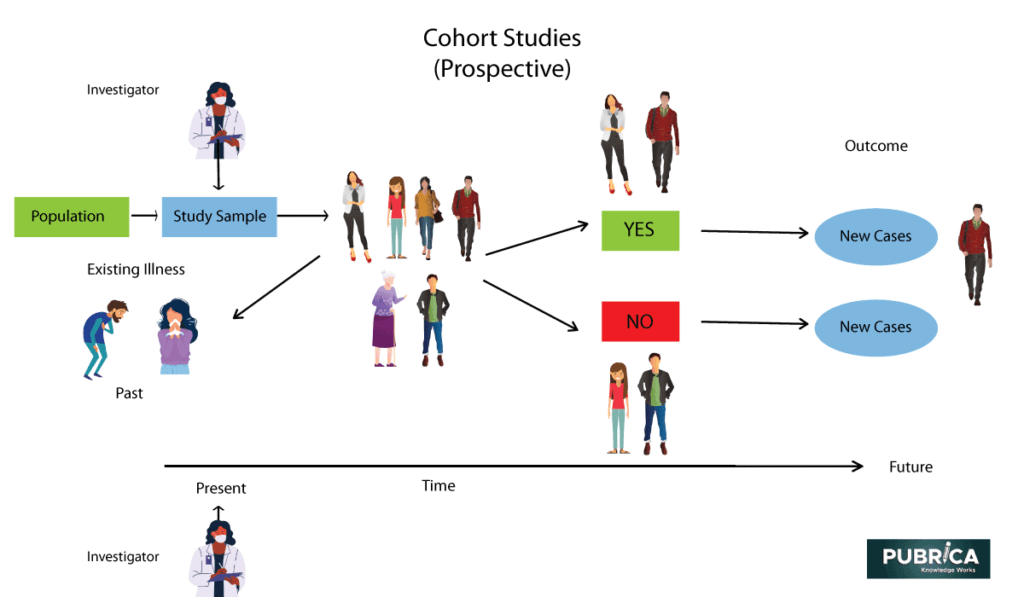
Cohort Study design
Patients in cohort studies are initially divided into two classes based on their contact status. The Cohorts are followed over time to see who in the exposed and non-exposed classes develops the disease. Retrospective or prospective cohort studies are both possible. In contrast to a case-control study, which starts with diseased and non-diseased patients, a cohort study starts with exposed and unexposed patients, allowing for direct calculation of incidence. A cohort study’s impact is calculated using relative risk. Recall bias is very minimal in cohort studies, and many results can be studied at the same time. Cohort studies have the drawback of being more susceptible to selection bias. Cohort research can be very costly and time-consuming when researching rare diseases and results with long follow-up periods.
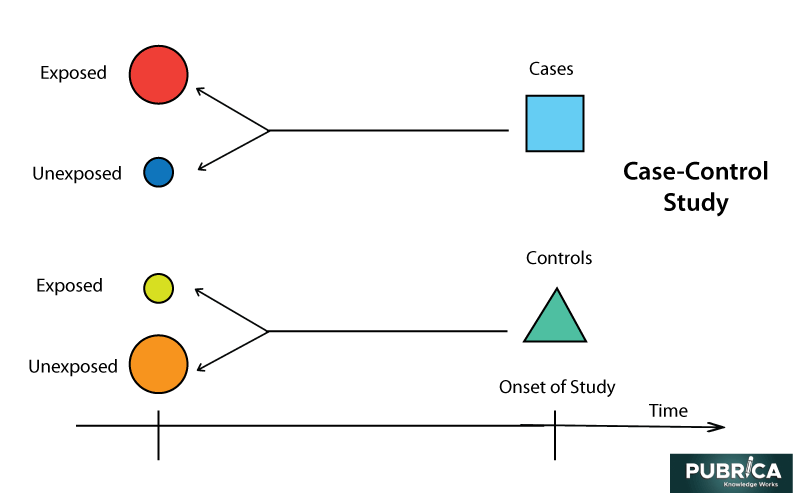
Case-Control Studies
Contrasted with the cohort and cross-sectional studies, case-control considers are generally retrospective. Case-control contemplates easy to arrange and reflectively contrast two gatherings with discovering the indicators of a result. Grant appraisal of the impact of indicators on the result utilizing the count of a chances proportion.
Cross-Sectional Studies
Cross-sectional studies are retrospective and include a snapshot of the research subjects’ characteristics at a specific point in time. Cross-sectional studies, unlike cohort studies, do not require a follow-up period and are thus relatively easy to perform. The weakest of the observational designs, cross-sectional research design, cannot include cause-effect relationships. The exposure status and result of interest information are obtained in a single moment in time, often through surveys. This method is often used to define the prevalence of a disease in a population.
Ecological Studies
Data at the person level is inaccessible, or large-scale comparisons are needed to investigate the population-level impact of exposures on a disease condition. Ecological studies are used. As a consequence, ecological research findings are only valid at the community level. In ecological studies, the types of measures used are aggregates of individual-level data. As a result, these studies are prone to a form of confounding known as an ecological fallacy, which arises when associations found in group data are presumed to hold for individuals. In public health science, ecological experiments are commonly used.
Experimental Study Designs
Randomized Clinical Trials
The gold standard in research design is randomized clinical trials, also known as randomized control trials (RCT). In an RCT, the participants are randomly assigned to one of two groups: control or experimental. Randomization eliminates confounding and reduces selection bias in RCTs. This allows the researcher to establish identical experimental and control groups, allowing them to isolate the intervention’s influence. The experimental group is exposed to/treated with a drug that may involve the cause, prevention, or treatment of a disease. The groups are then followed in the future to see who develops the desired outcome. RCTs are costly, and researchers who use this study often encounter problems with randomization integrity due to refusals, dropouts, crossovers, and non-compliance.
Conclusion
There are numerous potential wellsprings of errors that can bring about distortions of study results. These bends are an issue, particularly when the disease transmission specialist assesses the relationship between a risk factor and a medical condition. Whether a risk factor or a defensive factor goes undetected, or typical conduct or condition is misidentified as a risk or defensive factor, the ramifications may bring genuine ramifications for general society. A mistakenly recognized danger component may cause superfluous dread among people, or perhaps an unnecessary redirection of the restricted investigation reserves. Disease transmission specialists leading observational examinations (cohort, go-sectional, and specifically case-control should be aware of predispositions’ ability and apply extra consideration to eliminate or cut their result. As a translator of reports, we, the overall population, must be aware of the suitable predispositions in such reviews after we outline their decisions as recommended by the mass communications.
References:
- Chidambaram, A. G., & Josephson, M. (2019). Clinical research study designs: The essentials. Pediatric Investigation, 3(4), 245-252.
- Puljak, L., Makaric, Z. L., Buljan, I., & Pieper, D. (2020). What is a meta-epidemiological study? Analysis of published literature indicated heterogeneous study designs and definitions. Journal of comparative effectiveness research, 9(7), 497-508.
- www.researchgate.net
- Rezigalla, A. A. (2020). Observational study designs: Synopsis for selecting an appropriate study design. Cureus, 12(1).
