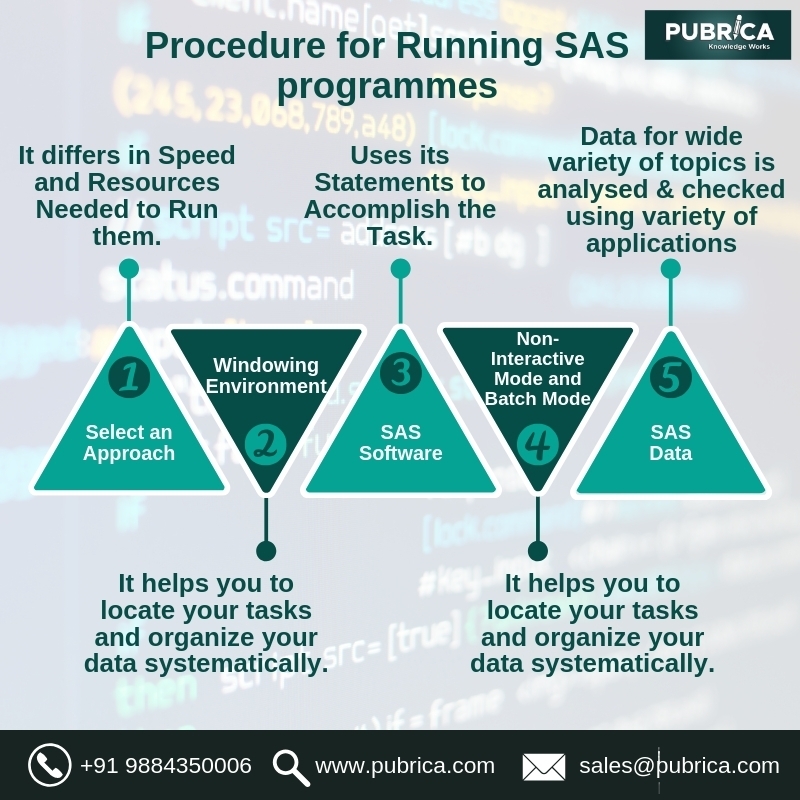
The Encodings of class variables in SAS regression procedures:
August 31, 2019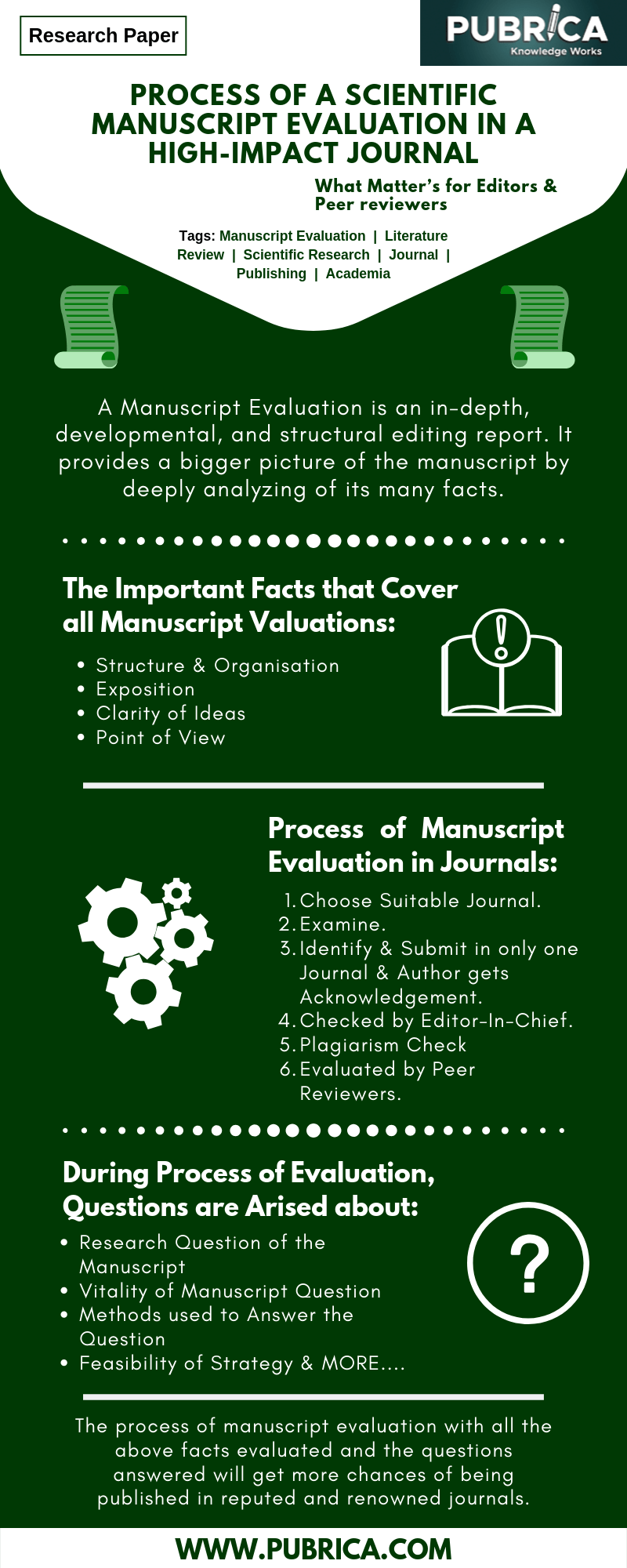
The Process of a scientific manuscript evaluation in a high-impact Journal – What Matter’s for Editors & Peer reviewers
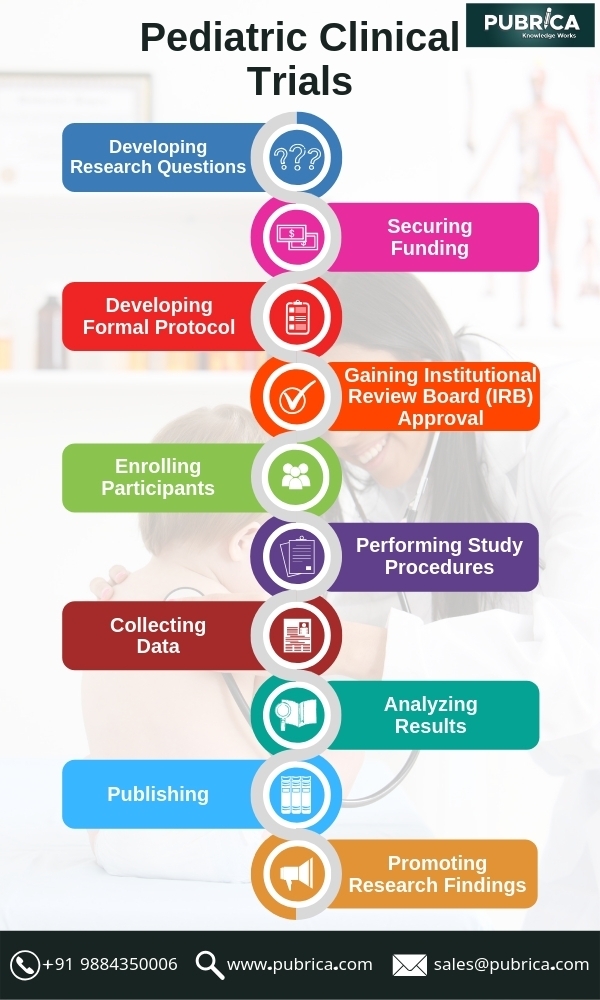
October 1, 2019In pediatric clinical trials, there are usually difficulties including a small number of patients and researchers, inclusion/exclusion criteria to further decrease the number of patients and a competitive study landscape generated by pediatric regulatory for children. Innovative methods are required to overcome these difficulties.
Children are still challenged to enrol in pediatric clinical trials. More efficient strategies are required to enhance child recruitment. Parents’ priorities and considerations must be a central focus, beginning with initial trial design in order to make the clinical trial accrual to be successful. The challenges can be addressed by expanding the number of sites or countries for adult clinical trials; however, due to variable regulatory environments and a dearth of appropriately skilled or interesting sites this approach is unrealistic in pediatric trials. Site accessibility is often hindered by a perception that pediatric trials are not scientifically stimulating or it gives a low return on investment rates because of the low amount of patients being registered each year. A multifactorial approach, including conducting advance feasibility assessments, utilizing pediatric networks, collaborating across institutions, and using alternative statistical approaches is required for improving the practicability of pediatric trials.
Clinical research Services involves investigating proposed medical treatments, assessing the relative benefits of competing therapies, and establishing optimal treatment combinations.
Bayesian statistical analysis is used to improve pediatric trial feasibility, using pediatric Type-2 diabetes is one such example. Using Bayesian data analysis, the potential to decrease the number of subjects required for a pediatric trial is demonstrated. The enrollment into pediatric trials for this indication is particularly challenging hence type 2 diabetes is chosen, and the disease pathophysiology is considered to be similar in adolescents and adults, making it a good candidate for this statistical approach. [2]
In medical research, the Bayesian method is increasingly used. The flexibility of the strategy from Bayesia enables the construction of clinical trial models with excellent characteristics of all kinds. Examples include maximization of efficient therapy for patients in the trial, maximization of dose-response curve slope data, cost minimization, minimization of the number of patients treated and minimization of the duration of the test. Clinicians can be more comfortable in assessing, developing and conducting clinical research by understanding these two statistical methods.[2]
Clinical Data management services in clinical research statistics involve the process of data compilation & clean-up, organizing of clinical data in compliance with good clinical data management practices as well as appropriate regulatory requirements. The basic goal of the Clinical Data Management (CDM) method is to provide superior information by decreasing mistakes in data entry and to prevent negligible information as far as possible. To accomplish this goal, best practice in clinical data management is implemented for the purpose of ensuring extensive, coherent and thorough information control.
The reliability of records of complete data shared between different medical experts for better treatment of patients is endured by the automation of clinical data management. The patient and medical professionals focus largely on clinical data management in the health industry with real-time evidence.[2]
Statistical analysis is one of the foundations of evidence-based clinical practice, a key element in the conduct of new clinical research and in the evaluation and implementation of prior research. Clinical research statistics shall include statistical power analysis and sample size planning, and the selection and conduct of appropriate analyzes in the light of the sampling and measurements used.[3]
Clinical Decision Support Systems leverage analytics in clinical trials and use the predictive analysis technology to collect and analyze individual patient information that serves as a guideline for future care strategies. This includes rule-based, encoded expert opinions, mathematical models for risk assessment and neural network models for accurate statistics.
The goal of clinical trials predictive analytics is towards helping the hospitals transform data into actionable insights that can help in improving business decisions. Improved competition globally, as well as the requirement for sustainable growth, are approaching more and more companies towards adapting analytical techniques for business insights. Healthcare organizations are seen more than ever to use analytics to consume, identify and apply new information insights.
Innovative analytical methods are used to drive clinical and operational improvements to meet business challenges.[3]
The following summarizes how predictive analytics benefits different segments of the healthcare industry:
- Life-sciences: Clinical research and drug discovery aid.
- Healthcare providers: Diagnostic assistance and clinical decision support aid.
- Insurance providers: Help in the prevention of fraud and optimizing healthcare costs.
- Public health: Help in identifying epidemic outbreaks as well as monitoring public health status.
- Individuals: Help in critical care intervention as well as monitoring public health status.
Reducing the size of the test by using Bayesian statistics would allow pediatric studies to be completed so that drugs can be appropriately marked with children. Hence this strategy may boost the use of Bayesian Statistical methods in pediatric clinical research, which will eventually enhance the opportunity of pediatric clinical trials that convince caretakers and finally enhance pediatric care.[3]
References :
[1] Robin A. Huff et al(2017), ” Enhancing pediatric clinical trial feasibility through the use of
Bayesian statistics”, International Pediatric Research Foundation, Inc.,16 August 2017.
[2] Bradley P. Carlin et al. (2017),” Statistical modelling for Bayesian extrapolation of adult clinical trial information in pediatric drug evaluation”, Pharmaceutical statistics 2017.
[3] Roger J Lewis et al. ” An introduction to the Bayesian analysis of clinical trials”, Sciencedirect.
Tags:
Biostatistical Programming | Clinical trials | journal Publishing services | Scientific Editing Services | Medical Writing Services | scientific research writing service | Scientific Medical communication service
Related Topics:
Literature gap and future research