Various ways in the prevention of fatal Fever of unknown origin
April 24, 2020
Uses of artificial intelligence (AI) in measuring the impact of research
May 2, 2020In Brief
Clinical epidemiology aims at preventing, detecting and treating diseases in an efficient manner. John R Paul coined the term “clinical epidemiology” about fifty years ago. Clinical epidemiology focuses on medicines to improve health, especially in the present world, when individuals are encouraged to take care of their own health. Here, a population is chosen to study the health related phenomena of different diseases. Either a patient population could be chosen or some other community based population can also be considered which has the conventional numerator and denominator values of an epidemiological study(Ehrenstein et al., 2017).The image given below illustrates what is done in clinical epidemiology:
Figure 1:
The distribution and determinants of diseases in human population is investigated in these studies and clinical epidemiology serves as the right tool in this respect. However, clinical epidemiology cannot be considered as an independent science because several skill sets are required in this field such as biostatistics, health social science and clinical economics. A gold standard testing procedure is used in clinical epidemiology. Results are the strength of relationship between two events which are measured through ratios, rates and proportion tests in clinical epidemiology. This relationship data is presented in a table wherein the number of false positive and true positive diagnostic test results is visualized. This table also gives an idea about the number of patients actually having and not having this disease(Huang et al., 2020).
Figure 2:
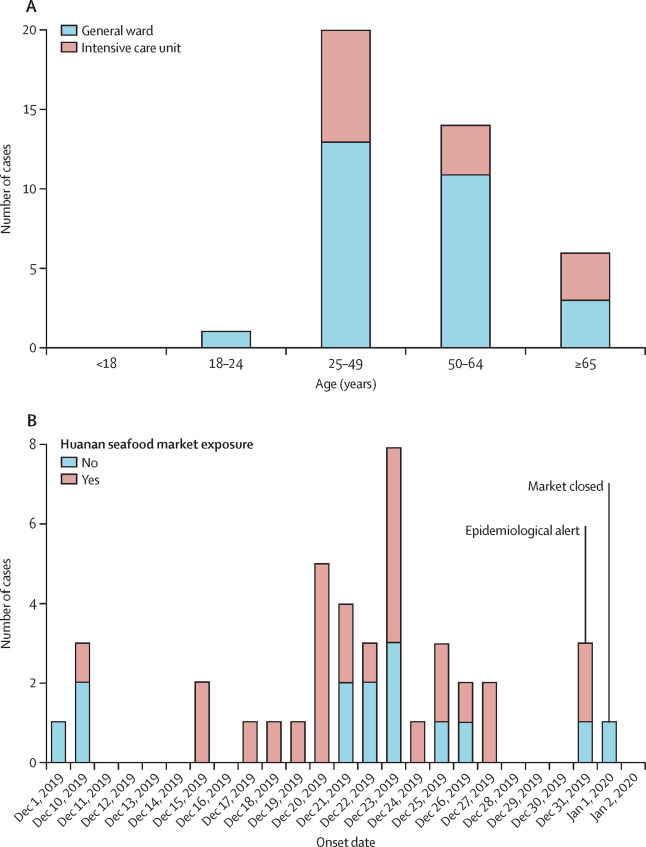
Figure 3:
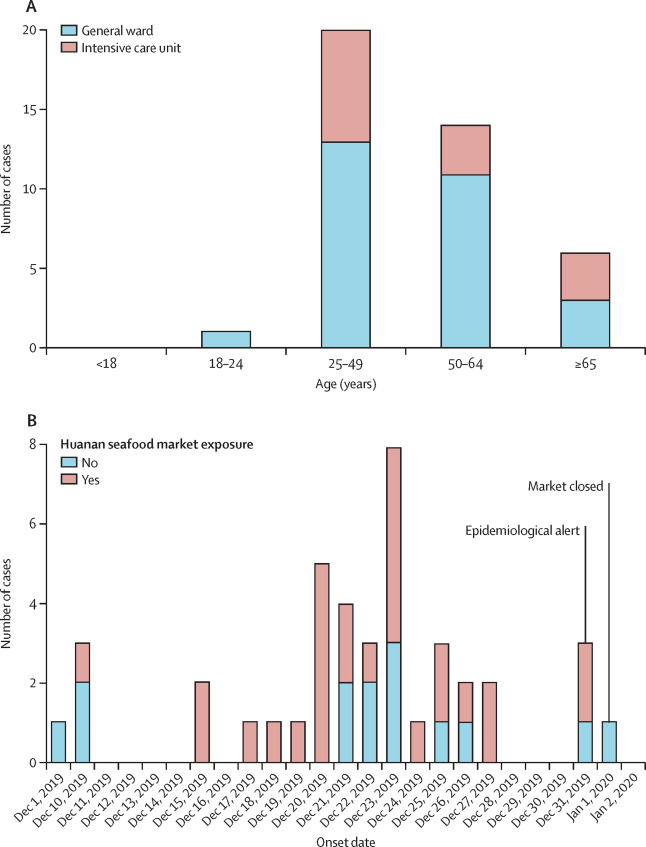
Given below are a few figures illustrating clinical epidemiology study results of patients suffering from Covid-19 in Wuhan, China. The graphs depict their clinical features.
Clinical epidemiology studies three kinds of diseases:
- Endemic disease – it affects the population at a relatively constant and expected rate within a specific region. The spread of pathogens and vectors, which are the reservoirs of disease is the main cause of an endemic disease. Geographical features, climate changes, poor living conditions such as dull houses, improper sewage systems and hazardous work conditions are other responsible factors for an endemic disease. Example includes Malaria in Africa.
- Epidemic disease -the population is affected at an unusually fast or unexpected rate.It occurs due to the increase in infectivity of a pathogen, poor living conditions such as crowded areas and improper sanitation systems, spreading and introduction of the pathogen in a new geographical condition. Example includes Ebola fever in West Africa.
- Pandemic – it is defined as a worldwide epidemic, the reasons being global trade and worldwide travelling and the increased infectivity of pathogens due to antigenic shift and gene mutations. Example includes Covid-19.
Clinical epidemiology calls in for clinical research, so that the population affected in the epidemic get the required treatments to eradicate the disease as fast as possible(Adhikari et al., 2020).
Clinical research is defined as “a careful study and an investigation in order to discover new facts and information”. Unbiased, reliable and valid measurements are required to carry out a clinical research programme. Clinical research addresses a specific question in an organized and objective manner(Magnin et al., 2019). The different types of clinical researches are described below.
- Treatment research : It involves an intervention such as medication, psychotherapy, new devices, new approaches to surgery and radiation therapy
- Prevention research: Finds out better ways to prevent the development or the return of diseases. Medicines, vitamins, vaccines, minerals and lifestyle changes are usually studied here.
- Diagnostic research: This refers to the practice to look out for better ways for the identification of a particular disorder or condition
- Screening research: It aims to find the best possible ways for the detection of particular disorders or typical health conditions
- Quality of life research: This explores ways to improve comfort levels and the quality of life for individuals suffering from a chronic illness
- Genetic studies: These studies aim to improve the prediction of disorders through identification and understanding of the relationship between genes and ill health. The different ways in which genes of an individual is more likely to develop a disorder is explored in this research domain. This in turn leads to the development of tailor-made treatments which are based on a person’s genetic make-up
- Epidemiological studies:They seek to identify the patterns, causes and control of disorders in different groups of people. “Outpatient” clinical research requires that participants do not stay overnight at the treatment centre. “Inpatient” clinical research requires the participants to stay for at least one night in the hospital or research centre.
Given below is an illustration of clinical research study results on the causes, clinical manifestation, epidemiology, control and prevention of Covid-19 at the outbreak of the disease(Magnin et al., 2019):
Figure 4:
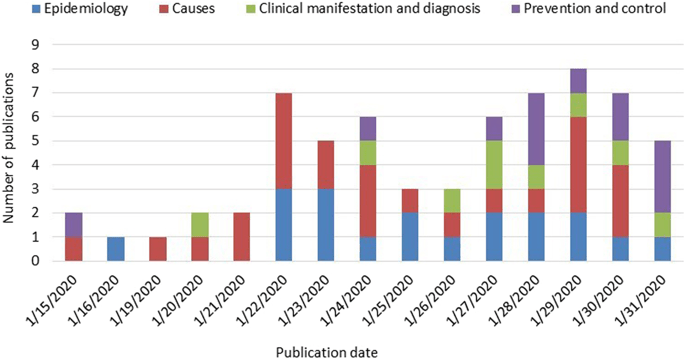
Clinical research involves conducting of clinical trials. These are done in four phases:
- Phase I: An experimental drug or treatment is tested in a small group (about 20-80) of people for the first time. The safetyof treatment is determined by the researchers and they suggest a safe dosage range of the drug, with the identificationof side effects
- Phase II :The experimental drug or treatment is given to a larger group (about 100-300) of people to see if it is effective and to further evaluate its safety
- Phase III:The experimental drug or treatment is given to large groups (about 1000 -3000) of people. The effectiveness is confirmed, side effects are monitored, and these are compared to commonly used treatments. Information is collected that allowssafe use ofthe experimental drug or treatment
- Phase IV: Post marketing studies are conducted after a treatmentis FDAapproved.It provides additional information about the risks, benefits and best usage of the drug or treatment.
Conclusion
The factors considered in a clinical research studyinclude age, gender, underlying disease and health history. Clinical trials are carried out in hospitals, clinics, individual physician’s chambers, university health centres and community health centres, and patient privacy is essentially maintained during any clinical trial.
References
- Adhikari, S.P., Meng, S., Wu, Y.-J., Mao, Y.-P., Ye, R.-X., Wang, Q.-Z., Sun, C., Sylvia, S., Rozelle, S., Raat, H. & Zhou, H. (2020). Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infectious Diseases of Poverty. [Online]. 9 (1). pp. 29. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-020-00646-x.
- Ehrenstein, V., Nielsen, H., Pedersen, A.B., Johnsen, S.P. & Pedersen, L. (2017). Clinical epidemiology in the era of big data: new opportunities, familiar challenges. Clinical Epidemiology. [Online]. Volume 9. pp. 245–250. Available from: https://www.dovepress.com/clinical-epidemiology-in-the-era-of-big-data-new-opportunities-familia-peer-reviewed-article-CLEP.
- Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., Cheng, Z., Yu, T., Xia, J., Wei, Y., Wu, W., Xie, X., Yin, W., Li, H., Liu, M., Xiao, Y., Gao, H., Guo, L., Xie, J., Wang, G., Jiang, R., Gao, Z., Jin, Q., Wang, J. & Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. [Online]. 395 (10223). pp. 497–506. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620301835.
- Magnin, A., Iversen, V.C., Calvo, G., Čečetková, B., Dale, O., Demlova, R., Blasko, G., Keane, F., Kovacs, G.L., Levy-Marchal, C., Monteiro, E.C., Palmisano, L., Pella, D., Portolés Pérez, A., Rascol, O., Schmid, C., Tay, F., von der Leyen, H. & Ohmann, C. (2019). European survey on national training activities in clinical research. Trials. [Online]. 20 (1). pp. 616. Available from: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-019-3702-z.



