Methodological Framework of SPIRIT Checklists?
The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement was issued in 2013 as “evidence-based guidelines” for the minimal information that should be included to describe a randomised controlled trial (RCT) protocol. Trials, like many other journals, supported and implemented the checklist, mandating any unstructured protocols published in Trials have a complete SPIRIT checklist.
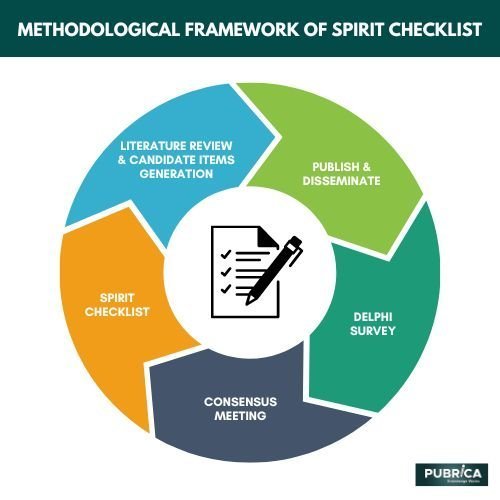
It is critical to have very defined RCT methods. The reader must be able to quickly grasp the trial approach and what is planned. They must be aware that processes are in place in the case of protocol violations and protocol changes, loss to follow-up and missing data, and how solicited and spontaneously reported adverse occurrences are handled with. This design is critical for the experiment and the results that will be released once the data has been analyzed. After all, participants have agreed to take part in these studies, and their time and well-being are important. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) gives recommendations for structuring RCT protocols and guarantees that all necessary information is included. Unfortunately, not all trialists follow the guidelines, and occasionally the material is misconstrued. Pubrica has provided information to assist authors, peer reviewers, editors, and other existing and prospective SPIRIT protocol editors in using and understanding the SPIRIT guidelines, based on their experience peer-reviewing for Trials during the previous two years. The guidance in this part from experienced protocol editors should assist writers and editors in ensuring that their publications comply with the SPIRIT statement requirements(1).
