
How to write a response to the reviewer for your systematic review?
June 20, 2022
Cross-sectional Research on Professional Medical Writing Support and the Standard of Reporting for Randomized Controlled Trials?
September 2, 2022How to conduct a systematic review for prospective studies?
The purpose of this systematic review is to consolidate and critically assess the findings of prospective research from many fields. Prospective studies can provide important information on cause-and-effect associations, risk factors, and prognostic variables. A thorough search of electronic resources and manual assessment of references discovered prospective studies fulfilling the inclusion criteria. This blog gives a thorough review of performing prospective studies as well as recommends interesting areas for additional research.
Introduction
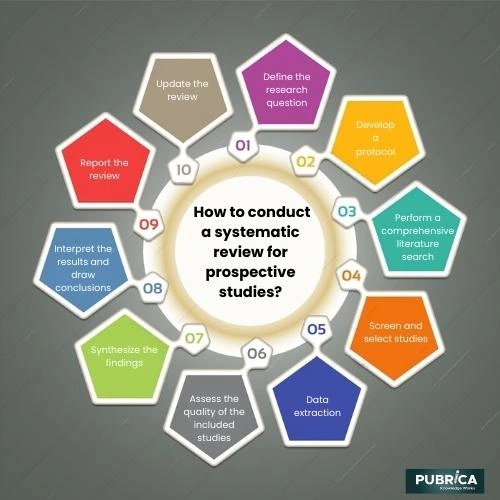
A systematic review of prospective studies involves a rigorous and structured approach to identifying, appraising, and synthesizing all the relevant research on a specific topic. Here is a step-by-step guide on how to conduct a systematic review of prospective studies:
- Define the research question: Start by developing a clear and focused research question, specifying the population, intervention or exposure, comparator, and outcome (PICO) elements.
- Develop a protocol: Before starting the review process, create a protocol that outlines the objectives, inclusion and exclusion criteria, search strategy, data extraction process, and methods for assessing study quality and synthesizing findings.
- Perform a comprehensive literature search: Conduct a thorough search of relevant databases (e.g., PubMed, Embase, Web of Science) using appropriate keywords and search terms. Additionally, check reference lists of included studies and relevant reviews for potentially eligible studies.
- Screen and select studies: Screen the titles and abstracts of the identified articles for eligibility according to the pre-defined inclusion and exclusion criteria. Obtain the full text of potentially eligible articles and perform a more detailed assessment to determine their suitability for inclusion in the review.
- Data extraction: Extract relevant data from the included studies using a standardized data extraction form. This may include study design, population characteristics, sample size, intervention or exposure details, outcome measures, and results.
- Assess the quality of the included studies: Evaluate the quality and risk of bias of the included prospective studies using an appropriate appraisal tool (e.g., the Newcastle-Ottawa Scale for cohort studies or the Cochrane Risk of Bias tool for randomized controlled trials). This step helps ensure that the review findings are based on high-quality evidence.
- Synthesize the findings: Depending on the included studies’ nature and heterogeneity, you may perform a meta-analysis to quantitatively combine the results or conduct a narrative synthesis to describe the findings qualitatively. In both cases, report the main results, including effect estimates and measures of uncertainty (e.g., confidence intervals).
- Interpret the results and draw conclusions: Discuss the review’s main findings in the context of the existing literature, considering the strength and limitations of the evidence. Provide recommendations for clinical practice or policy and suggestions for future research.
- Report the review: Write a clear, concise, and well-structured report of the systematic review, following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to ensure transparency and completeness.
- Update the review: Systematic reviews may need to be updated periodically as new research becomes available. Keep track of new evidence and consider updating the review if significant findings emerge that might change the conclusions.
Remember, a systematic review of prospective studies requires a rigorous and transparent approach to ensure the validity and reliability of the findings. If possible, collaborate with a team of reviewers to minimize bias and errors throughout the review process.
- To know more about systematic review Services, check our study guide. How to write a systematic review manuscript?
Research question and objectives
Systematic review research questions should be practical, intriguing, new, ethical, and relevant. PICO (Population, Intervention, Comparison, Outcome) and SPIDER (Sample, Phenomenon of Interest, Design, Evaluation, Research type) are two often utilized tools. PICO is utilized for quantitative evidence synthesis and is more sensitive than the specialized SPIDER technique. SPIDER is recommended for qualitative and mixed techniques searches. A hybrid strategy employing both methods is advised for extensive searches, especially when applying qualitative research to qualitative issues.
PICO is commonly used for clinical trial studies, systematic reviews, and meta-analyses. P (Patient) and O (Outcome) are used to design research questions in observational studies. The depression/anxiety screening and impact on COPD exacerbations severity, for example, is being developed and is now through phase I, II, and III clinical trials. The study topic should be based on PICO in order to establish the safety and immunogenicity of the severity of COPD exacerbations in humans.
- Check our systematic review Servicesample work to know and learn more about “ A Systematic Review of depression/anxiety screening and impact on COPD exacerbations severity.“.
Inclusion and exclusion criteria
The PICO technique, research design, and date are used to determine eligibility. The most common exclusion criteria include irrelevant, duplicated, unavailable full texts, or abstract-only studies. These exclusions should be communicated in advance to avoid bias in the researcher. Articles containing the target patients, researched interventions, or a comparison of two studied therapies would be the inclusion criteria. In a nutshell, it would be publications that include information that answers our study topic. The most significant aspect is that there should be clear and adequate positive and negative information to answer the question.
Conclusion
In conclusion, conducting a systematic review for prospective studies is a meticulous and essential process ensuring research findings’ reliability and validity. The key to success lies in adhering to a well-structured methodology that includes defining the research question, developing a comprehensive search strategy, screening studies based on pre-defined criteria, and critically appraising the selected articles. By synthesizing the data from multiple high-quality prospective studies, systematic reviews provide a robust and unbiased overview of the evidence, which can inform clinical decisions, policy-making, and future research directions. However, it is crucial to acknowledge potential limitations and biases during the process and interpret the results cautiously. Through rigorous adherence to these guidelines, systematic reviews can significantly contribute to evidence-based practice and advance our understanding of various research topics.
About Pubrica
Pubrica’s team of researchers and authors develop Scientific and medical research papers that can be an indispensable tools to the practitioner/authors. Pubrica medical writers help you to write and edit the introduction by introducing the reader to the shortcomings or empty spaces in the identified research field. Our experts know the structure that follows the broad topic, the problem, and the background and advance to a narrow topic to state the hypothesis.
References
- Tawfik, G.M., Dila, K.A.S., Mohamed, M.Y.F. et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health 47, 46 (2019).
- Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. J R Soc Med. 2003 Mar;96(3):118-21. doi: 10.1177/014107680309600304

14 years of expertise in clinical research with a doctoral distinction in life science.


