
In UK, an observational investigation on vitamin D and COVID-19 risk for Medical Research
January 17, 2022
A review of current research on the influence of open access to the scientific literature
January 21, 2022Introduction
WHAT ARE REPORTING GUIDELINES?
Reporting guidelines are tools that advise authors publishing a scientific article on particular study items to be disclosed to improve the research rigour, reproducibility, transparency, and scientific community acceptance of the study results and conclusions. Reporting guidelines usually outline the development process and give researchers a checklist of elements that should be reported based on the study design. The checklist is extremely valuable since it offers writers an easy-to-follow structure for planning the research endeavour, from study protocol preparation to study implementation, data analysis, and manuscript writing. Each research design has its reporting standards (Table 1). The Enhancing the Quality and Transparency of Health Research (EQUATOR) Network, a global project aimed at improving published health research reporting quality, has established the most widely used reporting guidelines. The most well-known EQUATOR recommendations are Consolidated Reporting Trials (CONSORT) for randomised clinical trials (RCTs) and STROBE for observational research. Several offers share specific components, such as the experimental research design in the paper title and the participant flow diagram, which shows how many people were screened for eligibility, how many were eliminated, and why they were excluded. Other factors to consider are unique to each research design (e.g., the type of randomisation procedure used in RCTs within the CONSORT guideline) (1)
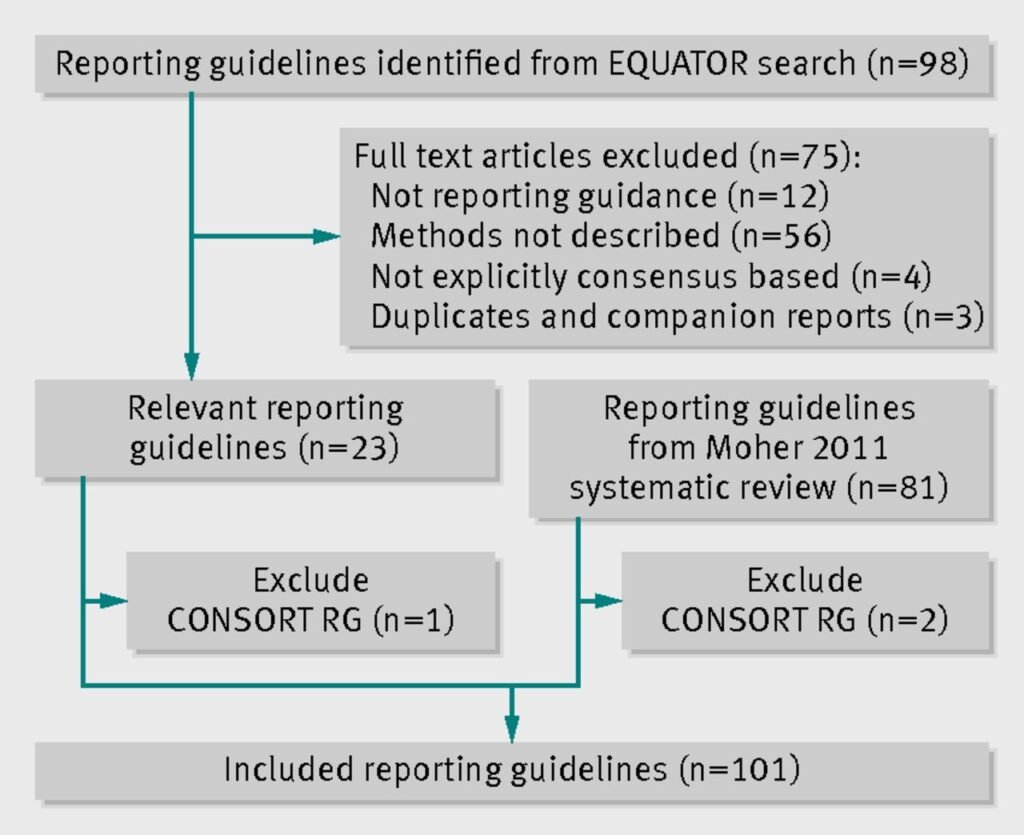
Figure. 1 Reporting guidelines of EQUATOR(2)
In the context of this systematic review, definitions relevant to the evaluation of reporting guidelines
Endorsement— a journal’s action to show that it supports using one or more reporting guideline(s) by authors submitting research reports for review; generally accomplished through a statement in its “Instructions to authors” section.
Adherence— Action did by an author to ensure that a manuscript complies with the items indicated by the appropriate/relevant reporting guideline (reports all specified items).
Implementation— Journals are taking steps to guarantee that authors follow approved reporting criteria and that published articles are fully documented.
Complete reporting— the status of a research report’s reporting and whether it complies with any applicable reporting standard.

Scenario
Researchers conducted a prospective cohort analysis of 101 patients admitted to the ICU at a university hospital in So Paulo, Brazil, and analysed mechanical ventilator waveforms to quantify the prevalence of patient-ventilator asynchrony. According to the researchers, a high majority of asynchrony was linked to more significant weaning failure, but not to death. The study results were published using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting criteria (3).
WHY ARE REPORTING GUIDELINES IMPORTANT?
The use of reporting rules ensures that writers disclose all essential components of a research study, allowing the reader to comprehend all relevant parts of the investigation properly. This is critical because other researchers may duplicate methods. Results can be included inefficient reviews or utilised by clinicians to assist clinical decision making when a paper provides accurate and comprehensive study information. For example, when a paper publishes the results of an RCT but fails to mention how many prospective participants were removed from the study, the generalizability and internal validity of the findings may be compromised. Similarly, in our supposed situation, if the publication omitted to indicate how many participants were lost during follow-up, readers would be unable to assess the cohort study’s risk of bias. As a result, the findings would be useless in clinical decision-making. The international research community increasingly recognises that following reporting requirements enhances the quality of research and helps to reduce resource waste in poorly reported projects. As a result, most high-impact medical journals now require RCTs to follow CONSORT standards, and most observational studies incorporate STROBE flow diagrams (4).
Table 1. Reporting guidelines for most study designs.
| Study design | Reporting guidelines |
| Randomised trials | CONSORT |
| Observational studies | STROBE |
| Systematic reviews | PRISMA |
| Study protocols | SPIRIT, PRISMA-P |
| Diagnostic/prognostic studies | STARD |
| Prognostic studies | TRIPOD |
| Case reports | CARE |
| Clinical practice guidelines | AGREE, RIGHT |
| Qualitative research | SRQR, COREQ |
| Animal preclinical studies | ARRIVE |
| Quality improvement studies | SQUIRE |
| Economic evaluations | CHEERS |
About Pubrica
Pubrica’s manuscript editing services are second to none. Proofreading, which is the correction of grammar, spelling, and other surface problems and is the final step in the editing process, is sometimes mistaken with editing. The goal of scientific writing editing is to revise and organise the material of a document so that it is more succinct and exact. The method reduces idiom and eliminates wordiness, allowing for improved communication.
References
- Ferreira, Juliana Carvalho, and Cecilia M. Patino. “Reporting guidelines: essential tools for manuscript writing in medical research.” Jornal Brasileiro de Pneumologia 47 (2021).
- Stevens, Adrienne, et al. “Relation of completeness of reporting of health research to journals’ endorsement of reporting guidelines: a systematic review.” BMJ 348 (2014).
- Sousa MLA, Magrans R, Hayashi FK, Blanch L, Kacmarek RM, Ferreira JC. Predictors of asynchronies during assisted ventilation and its impact on clinical outcomes: The EPISYNC cohort study. J Crit Care. 2020; 57:30-35.
- Equator Network [homepage on the Internet]. Oxford: . Available from: UK EQUATOR Centre; [cited 2021 Feb 2]
