Biostatistics importance in clinical trial prevention, detection, and therapy
Biostatistics is essential for data collection, analysis, presentation, and interpretation in clinical research. Epidemiology, clinical trials, population genetics, systems biology, and other fields benefit. It aids in evaluating a drug’s efficacy and safety in clinical trials. The ability to think clinically and statistically is essential for medical advancement. Clinical researchers must generalize from a small number of cases to many cases, using empirical facts and theory. Empirical knowledge is derived from observations and data in medical and statistics sciences. Biostatistics is essential for data collection, analysis, presentation, and interpretation in clinical research.
The ability to think clinically and statistically is essential for medical advancement. Clinical researchers must generalize from a small number of cases to many cases, using empirical facts and theory. To create a hypothesis, you’ll need a biological theoretical basis and statistical support based on the observed data and the theoretical statistical model. A clinical trial is an experimental trial in which medical therapies are tested on humans. The clinical investigator controls elements that contribute to unpredictability and bias, such as subject selection, treatment application, outcome evaluation, and analysis procedures. The experimental nature of a clinical trial and its occurrence in humans distinguishes it from other sorts of medical studies.
Biostatisticians research novel approaches to ensure that policies, whether targeted at populations or individuals in need of care, are founded on the evidence of benefit.
- Interest therapy must be done naturally.
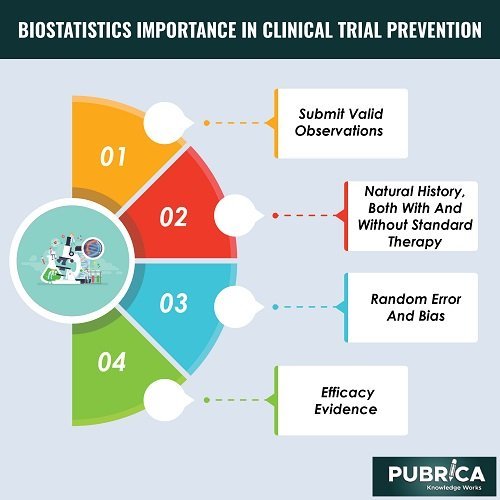
- The study participants must submit valid observations to answer the biological question.
- The condition’s natural history, both with and without standard therapy, must be known.
- The treatment’s effect must be substantial enough to compensate for random error and bias.
- Efficacy evidence must be based on biological understanding.
Reference:
- M Buyse 1, S L George, S Evans, N L Geller, J Ranstam, B Scherrer, E Lesaffre, G Murray, L Edler, J Hutton, T Colton, P Lachenbruch, B L Verma, The role of biostatistics in the prevention, detection and treatment of fraud in clinical trials Stat Med. 18(24):3435-51.
- What is the role of statistics in clinical research?
