Challenges, Strategies and Future Impacts in Healthcare
March 23, 2021
Use of ICMJE URM for Ethical Guidance
April 1, 2021Use of GPP3 for Ethical Guidance
GPP3 is a revised version of the Good Publishing Practice (GPP) guideline that offers guidelines for persons and organizations involved in disseminating research findings funded or assisted by pharmaceutical, medical device, diagnostics, and biotechnology industries. The guidelines are meant to help individuals and organizations adhere to legal and regulatory standards while ensuring fair and open publishing activities. These guidelines apply to peer-reviewed journal publications and oral and poster presentations at science conferences (2).
The Latest Subjects Discussed in GPP3
The following are some of the latest subjects discussed in GPP3:
- The most recent ICMJE authorship conditions are explained (2013)
- Authorship problems that are frequently found
- Author payment and repayment are now more explicitly described.
- Clarification of the words “ghost fiction” and “guest authorship.”
- Professional medical writers: what they do and how they help
- Advice on how to exchange data acceptably.

GPP Historical Archive
GPP2 Guidelines, 2009
International Society for Medical Publication Professionals (ISMPP) assembled a Steering Committee to produce an updated Good Publication Practice guide, now known as “GPP2,” to discuss regulatory, guidance, and ethical changes since 2003 and to affirm the goals of the initial 2003 publication(4).
GPP Guidelines, 2003
The critical Good Publication Practice (GPP) guidelines, released in 2003, aimed to ensure that “clinical trials funded by pharmaceutical firms are published responsibly and ethically.” (3)
GPP3 for ethical guidelines
The resulting guideline adds new parts (Principles of Good Publication Practice for Company-Sponsored Medical Research, Data Sharing, Studies That Should Be Published, and Plagiarism). It builds on authorship requirements and widespread authorship concerns established by the International Committee of Medical Journal Editors., Expand details on the position of medical writers and explain appropriate author payment and reimbursement. Individuals and organizations can embody honesty, transparency, and obligation for reliable, complete, and transparent reporting in their publications and presentations by adopting good publishing practices (including GPP3).
Role of ISMPP
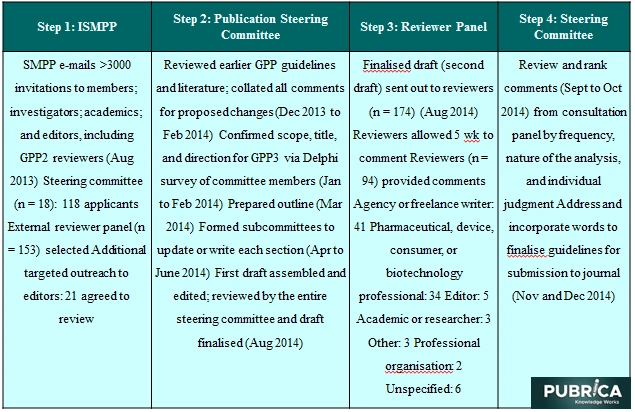
ISMPP was the energetic force behind the adoption of the GPP3 guideline. By supplying logistical research paper support, giving access to the mailing list of ISMPP participants, sending out e-mails to members and prospective reviewers, maintaining the database of respondents, setting up the reviewer Web site, and reviewing the GPP3 steering committee, the sponsor was able to assist in the formation of the GPP3 steering committee. Several members of the steering committee also sit on the ISMPP Board of Trustees. They did, however, contribute to GPP3 as entities, not on behalf of ISMPP. The quality of this guideline was not guided or regulated by the ISMPP employees.
Future Directions
We hope that GPP3 will add to the many valuable guidelines and recommendations that are already available (such as those from the American Medical Writers Association, Council of Science Editors, Committee on Publication Ethics, European Association of Science Editors, European Medical Writers Association, ICMJE, International Federation of Pharmaceutical Manufacturers & Associations), ISMPP, Medical Publishing Insights and Practices, and the World Association of Medical Editors) all promote responsible publishing practices and research(5).
Conclusion
Because of advancements in regulatory, medical, and journal standards, our understanding of the publishing environment has changed. The GPP3 guideline has been amended to keep up with the changes. The role, reimbursements, and increased responsibility of MWs in the process have been identified and elaborated to a greater extent. Necessitates MW staying current with current trends and undergoing specialised training from known institutions to improve understanding and formulate a proper strategy. It is required to produce high-quality documentation and the bridging of the gap between research conduct and reporting. Avail Publication support services from Pubrica.
References
- Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, et al. Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3. Ann Intern Med. 2015;163:461-464; doi:10.7326/M15-0288
- Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014;383:267-76. [PMID: 24411647] doi: 10.1016/S0140-6736(13)62228-X
- Wager E, Field EA, Grossman L. Good publication practice for pharmaceutical companies. Curr Med Res Opin. 2003;19:149-54. [PMID: 12814125]
- Graf C, Battisti WP, Bridges D, Bruce-Winkler V, Conaty JM, Ellison JM, et al; International Society for Medical Publication Professionals. Research Methods & Reporting. Good publication practice for communicating company sponsored medical research: the GPP2 guidelines. BMJ. 2009;339:b4330. [PMID: 19946142] doi:10.1136 /bmj.b4330
- Wager E, Woolley K, Adshead V, Cairns A, Fullam J, Gonzalez J, et al. Awareness and enforcement of guidelines for publishing industry-sponsored medical research among publication professionals: the Global Publication Survey. BMJ Open. 2014;4:e004780. [PMID: 24747794] doi:10.1136/bmjopen-2013-004780