- Home
- Insights
- Key Tips for Reviewing Systematic Reviews and Meta-Analyses
Important Tips for Reviewing Systematic Reviews and Meta- Analyses
Important Tips for Reviewing Systematic Reviews and Meta- Analyses

Dr.Nancy | Research design and Mixed Methods Research.
30 Jan, 2025

Dr.Nancy | Research design and Mixed Methods Research. 30 Jan, 2025
Introduction:
Reviewing systematic reviews and meta-analyses requires meticulous attention to various aspects, ensuring that the research is original, impactful, and methodologically sound. Adapted from guidelines presented on the BMJ website, this guide outlines key considerations for reviewers tasked with evaluating systematic reviews and meta-analyses [1].
1. Assessing Originality
- Determine whether the research adds new insights or builds upon prior work [2].
- Evaluate whether the systematic review and meta-analysis design appropriately addresses the research question [3].
- Search core databases (e.g., PubMed, Embase, CENTRAL) to identify similar previously published systematic reviews and meta-analyses [4].
- Justify the originality of the work by identifying differences from or updates to prior studies, such as the inclusion of recent data or divergent conclusions [1].[5]
2. Evaluating the Importance of the Work
3. Database Search Quality
Key Considerations:
- Confirm that multiple core databases were searched, including:
- PubMed (Medline)
- Embase
- CENTRAL (for RCT meta-analyses in medicine) [7].
- For observational studies (e.g., cohort or case-control studies), searching PubMed and Embase is typically sufficient.
- A single database search (e.g., only PubMed) is inadequate [8].
4. Inclusion and Exclusion Criteria
Key Considerations:
- Evaluate the study selection process using the PICO framework:
- Population (P): Target study participants.
- Intervention (I): Treatment or risk factor.
- Comparison (C): Control group or comparison.
- Outcome (O): Key measures of effect.
- Assess the appropriateness of inclusion criteria (e.g., study design) and exclusion criteria (e.g., duplicate data, non-English publications, or non-human studies).
5. Subgroup Analyses
Key Considerations:
- Examine whether subgroup analyses explore relevant factors, such as:
- Study quality (e.g., high vs. low-quality studies).
- Participant demographics (e.g., age, sex, race/ethnicity).
- Intervention characteristics (e.g., dosage, follow-up duration).
- Conflict of interest (e.g., pharmaceutical funding).
- Subgroup analyses can reveal significant associations even if the main meta-analysis does not [9].
6. Interpretation Based on Levels of Evidence
Key Considerations:
- Understand the hierarchy of evidence:
- Meta-analyses of RCTs provide the highest evidence level.
- Observational studies (e.g., cohort, case-control studies) are less robust.
- Ensure that mixed-study meta-analyses (e.g., combining cohort and case-control studies) are interpreted cautiously. Findings from higher-quality study designs should take precedence.
Example: If a meta-analysis shows a significant effect in case-control studies but not in cohort studies, the conclusion should favor the results of cohort studies due to their higher reliability.
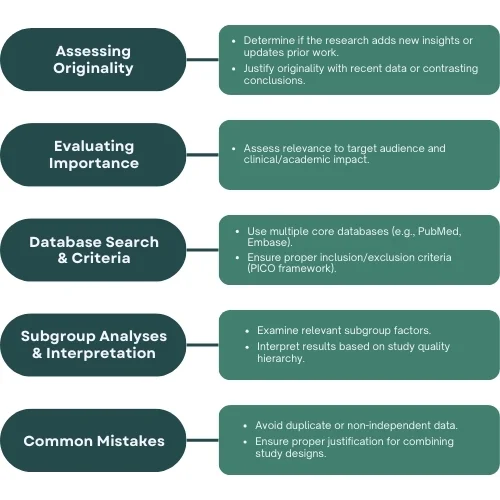
Figure 1: Important Tips for Reviewing Systematic Reviews and Meta- Analyses
7. Identifying Common Mistakes
Key Mistakes to Address:
- Duplicate Data: Ensure datasets appearing in multiple publications are not redundantly included.
- Non-Independent Data: Avoid combining overlapping participant data across studies.
- Inadequate Justification: Require explanations for combining diverse datasets or study designs.
Contact us today to learn how we can help you navigate the complexities of ethical publishing and prevent plagiarism in your research
We offer the expertise, knowledge, and comprehensive support your Clinical research and publication needs.
8. Comprehensive Evaluation of Manuscript Sections
Key Considerations for Each Section:
- Introduction:
- Does it clearly state the background, aims, and significance of the review?
- Methods:
- Are search strategies, inclusion/exclusion criteria, and analytic methods adequately described?
- Results:
- Are findings presented in clear, well-organized tables and figures?
- Do the results reliably address the research question?
- Discussion:
- Does it compare findings with prior studies?
- Are potential mechanisms and limitations adequately discussed?
- Conclusion:
- Is the conclusion concise and supported by the findings?
- References:
- Are the references current, relevant, and properly cited?
Conclusion
Reviewers play a critical role in ensuring the quality and reliability of systematic reviews and meta-analyses. By focusing on originality, importance, methodology, and interpretation, reviewers can help uphold the integrity of scientific literature. Following these comprehensive guidelines will facilitate a thorough and balanced evaluation, ultimately supporting the advancement of evidence-based medicine.
