From Lab to Literature: Exploring the Roadblocks Physicians Navigate in Publishing Their Research
January 18, 2024
Let’s Talk: Is it possible for physicians to maintain a balance between their clinical duties and research work?
January 30, 2024Understanding the need for meta-analysis
Meta-analyses have a significant advantage over individual studies. Researchers can combine data from multiple sources to increase statistical power, which leads to more precise estimates and higher confidence in the results. Also, meta-analyses allow researchers to analyze patterns and trends across studies, investigating possible sources of heterogeneity or inconsistency.
By combining the results of various studies, researchers can obtain a more reliable estimate of the true effect size or association. This approach helps to ensure that the conclusions drawn are trustworthy.
RCTs are considered the benchmark for evaluating the effectiveness of an intervention or treatment. By combining the results of several RCTs, meta-analyses offer a more accurate assessment of effectiveness. Therefore, meta-analysis and RCTs complement each other to provide a more reliable understanding of medical interventions.
The importance of randomized controlled trials (RCTs)
Randomized controlled trials (RCTs) are an important part of scientific research. They are highly respected for their ability to provide strong and dependable evidence. In the healthcare industry, for instance, RCTs are considered the most reliable method for evaluating the effectiveness of new treatments or interventions.
Randomized controlled trials (RCTs) are the most reliable way to establish causal associations in clinical research. There are several RCT designs and features that researchers can choose from to test their research hypotheses.[3]
The primary characteristic of a randomized controlled trial (RCT) is the arbitrary selection of participants into various groups. This randomization process aids in ensuring that the groups are similar in fundamental aspects, such as age, gender, and the severity of the condition. By reducing bias and confounding variables, RCTs enable researchers to confidently point out any differences in outcomes to the intervention being tested.
Randomized Controlled Trials (RCTs) are highly effective in establishing causality. By randomly assigning participants to different treatment groups, researchers can confidently attribute any changes in outcomes to the intervention itself rather than other factors. This is critical in determining whether a new drug, therapy, or intervention is truly effective and safe.
It has an edge when it comes to gathering measurable data that can be statistically analyzed. By collecting standardized data from participants in a controlled environment, researchers can apply statistical tests to determine the significance of any observed effects. This approach helps to measure the magnitude of the intervention’s impact. It provides a solid foundation for making fact-based decisions [1].
Researchers can compare the outcomes of the intervention group with a control group, which helps to identify any potential risks associated with the treatment. This information is decisive in ensuring patient safety and guiding clinical practice guidelines.
Harnessing the power of meta-analyses and RCTs for evidence-based decision-making
Meta-analyses offer a comprehensive overview of the existing research, enabling a more accurate assessment of the true effect size. This is particularly helpful when individual studies produce conflicting or inconclusive results. By combining data from multiple studies, meta-analyses can provide a more reliable estimate of the treatment effect.
Meta-analyses and randomized controlled trials (RCTs) are closely related, as meta-analyses usually involve analyzing data from several RCTs in a systematic and synthesized manner [2].
It aims to provide a comprehensive summary of the effectiveness and safety of a particular intervention. This is achieved by combining data from multiple randomized controlled trials (RCTs) to increase the sample size and statistical power. By doing this, more dependable conclusions can be drawn. To augment the RCTs with precision and reliability, go with Meta-Analysis Consulting and Outsourcing Services for expertise in transforming data into impactful research outcomes.
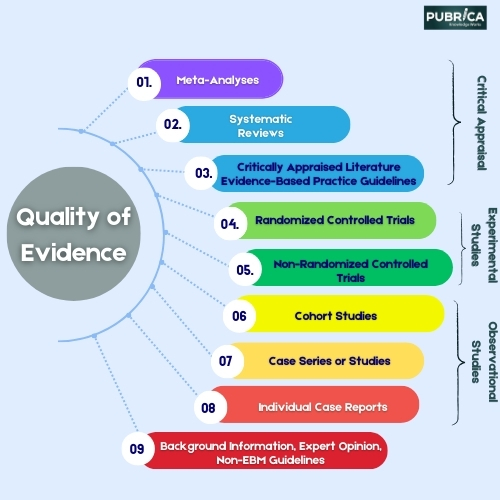
Meta-analysis consulting and outsourcing services for clinical trials include statistical analysis, data management, protocol development, literature search, result interpretation, and comprehensive reporting. These services provide support for researchers, pharmaceutical companies, and healthcare organizations interested in analyzing existing evidence from RCTs through a meta-analytic approach.
Such consulting and outsourcing services can help ensure that systematic reviews and meta-analyses are conducted following rigorous scientific standards, methodological guidelines, and reporting criteria (e.g., PRISMA). They can also help travel the complex process of identifying relevant studies, retrieving data, assessing study quality, and synthesizing results, improving the reliability and validity of the findings.
Pubrica offers exceptional meta-analysis services for observational studies, catering to the needs of researchers and scientists in the field. Trust Pubrica to bring out the true potential of observational studies through our meta-analysis services and boost the impact of your research.
In conclusion, as professionals in any field, it’s essential to understand the importance of aggregating data from multiple studies and the rigorous nature of randomized controlled trials. By doing so, we can make informed decisions and draw accurate conclusions. The use of meta-analyses can be a powerful tool for synthesizing evidence and informing evidence-based decision-making. Harnessing the power of meta-analyses is crucial, and it’s up to us to embrace this valuable tool to improve our professional practices.
References:
- Dechartres, Agnes et al. 2016. “Reporting of statistically significant results at ClinicalTrials.gov for completed superiority randomized controlled trials”. BMC Medicine 14(1): 192. http://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-016-0740-1.
- Sullivan, David J. et al. 2023. “Outpatient randomized controlled trials to reduce COVID‐19 hospitalization: Systematic review and meta‐analysis”. Journal of Medical Virology 95(12). https://onlinelibrary.wiley.com/doi/10.1002/jmv.29310.
- Zabor, Emily C., Alexander M. Kaizer, en Brian P. Hobbs. 2020. “Randomized Controlled Trials”. Chest 158(1): S79–87. https://linkinghub.elsevier.com/retrieve/pii/S0012369220304633.