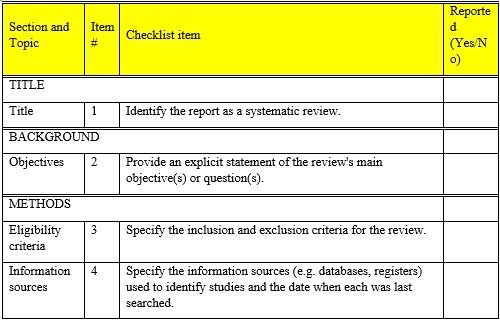
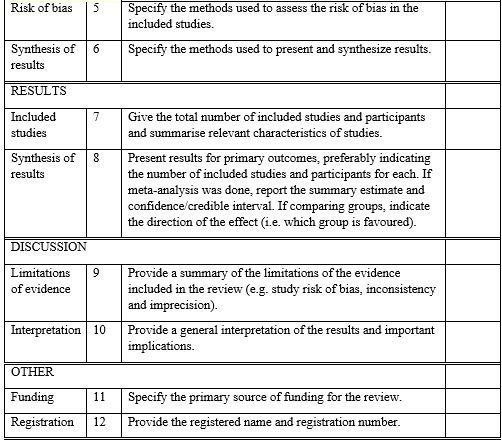
PRISMA 2020 Abstract Checklist
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, issued in 2009, was created to assist systematic reviewers in reporting why the review was conducted, what the authors performed, and what they discovered. Over the last decade, advances in systematic review methods and language have demanded an update to the guideline. The PRISMA 2020 statement supersedes the 2009 declaration and contains revised reporting recommendations that consider developments in approaches for identifying, selecting, evaluating, and synthesizing research. The items’ structure and appearance have been altered to enable implementation. We offer the PRISMA 2020 27-item checklist, an enlarged checklist with reporting suggestions for each item, the PRISMA 2020 abstract checklist, and redesigned flow diagrams for original and modified reviews in this publication. To promote widespread distribution, this paper is freely available on the websites of the journals BMJ, PLOS Medicine, Journal of Clinical Epidemiology, and International Journal of Surgery.
References
Page, M.J., McKenzie, J.E., Bossuyt, P.M. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10, 89 (2021). https://doi.org/10.1186/s13643-021-01626-4
