Model-based designs for dose-finding study
- In drug development, dosage-finding studies are critical because they establish a suggested dose for later-phase testing. For quicker and less expensive medication development, we need reliable, efficient phase I trial designs.
- Phase I studies utilise algorithm- or model-based approaches to identify a recommended dosage based on a target/acceptable toxicity threshold or other criteria. The maximum-tolerated dose (MTD) is the most significant dose of a medication or treatment that does not cause too many people to have undesirable adverse effects. Algorithm-based designs, such as the 3+3, identify the MTD and assign patients to a dosage level based on guidelines established during trial design. Patients’ information at one dosage level is used to allocate dose levels.
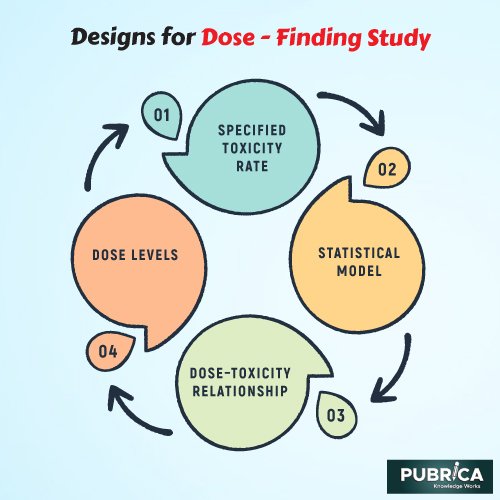
- Patients are assigned to a dosage level using a specified toxicity rate and a statistical model explaining the dose–toxicity relationship between dose levels in model-based designs like continuous reassessment (CRM). When a new patient joins the study, the model is updated using all available data on all existing patients. The new patient’s dose is decided using the model-suggested dose as a guideline. The next dose is determined using data from each patient at each dose level. At the end of the experiment, the model suggests the final MTD.
- There is a mountain of evidence that CRM is beneficial. Many of the world’s leading pharmaceutical corporations use model-based designs regularly. Pubrica has to replicate the advances made by the industry in trial design, and our study revealed hurdles for academic statisticians and clinical academics. Funders, regulators, and journal editors working together might lead to more precise dosages for later-phase testing, improving the speed and effectiveness of clinical drug development. In academia, we make suggestions for expanding the use of model-based designs for dose-finding studies.
References
Love SB, Brown S, Weir CJ, Harbron C, Yap C, Gaschler-Markefski B, Matcham J, Caffrey L, McKevitt C, Clive S, Craddock C, Spicer J, Cornelius V. Embracing model-based designs for dose-finding trials. Br J Cancer. 2017 Jul 25;117(3):332-339. doi: 10.1038/bjc.2017.186. Epub 2017 Jun 29. PMID: 28664918; PMCID: PMC5537496.
