Regular proper documentation of surgical cases promotes medical knowledge development while enhancing patient care quality. The Surgical Case Report (SCARE) standards introduced their format for surgical case report documentation in 2016 [1]. As surgical methods evolve, it becomes increasingly necessary to update medical guidelines.
- Home
- Insights
- Scare 2023 Guideliness
SCARE 2023 Guidelines: A Comprehensive Framework for Reporting Surgical Case Reports
Reference List Accuracy in Scholarly Writing : Common Errors and Best Practices



Noah Clarke | Associate Journal Editor 07,Mar 2025
Introduction
Regular proper documentation of surgical cases promotes medical knowledge development while enhancing patient care quality. The Surgical Case Report (SCARE) standards introduced their format for surgical case report documentation in 2016 [1]. As surgical methods evolve, it becomes increasingly necessary to update medical guidelines.
The SCARE 2023 rules from the study, “Sohrabi, C., et al. (2023)” represent significant development because they incorporate modern best practices as well as remedies to previous documentation concerns. The following article will provide guidance on the updated recommendations to help researchers, authors, and editors maintain consistent surgical literature quality through high-quality reporting [2].
Why Were the SCARE Guidelines Updated?
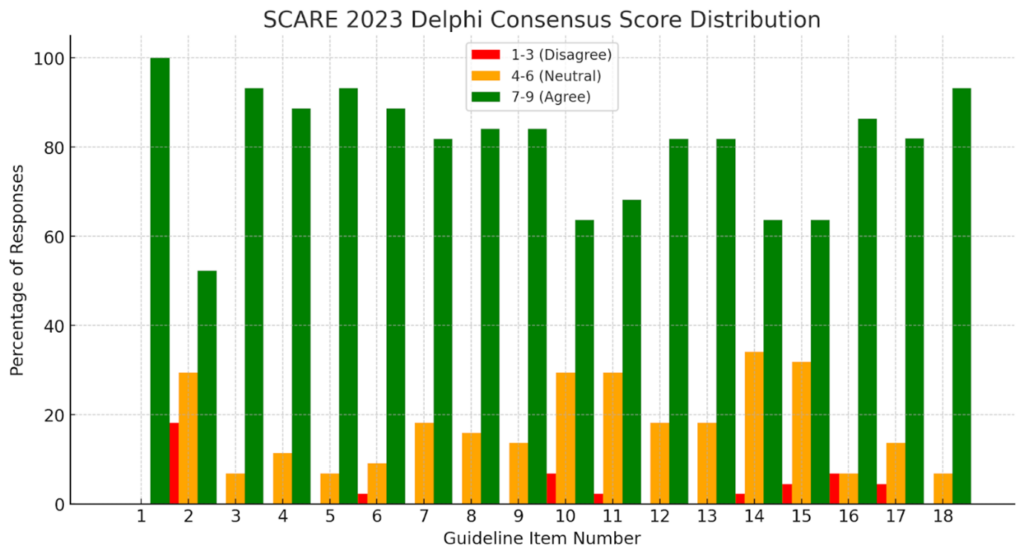
The Delphi process was utilized to rewrite the recommendations, resulting in a structured and methodical approach [4].
- The process includes inviting experts from the SCARE 2020 Delphi group, editorial board members, and peer reviewers.
- The experts accessed the survey platform through email communication to assess proposed improvements on the online platform.
- The survey used a 1 to 9 scale for statement evaluation requiring at least 70% agreement to include responses.
An 81.5% response rate emerged from 54 expert participants who finished their surveys among the 54 experts invited while 83.7% of 43 suggested improvements were accepted.
Key Updates in the SCARE 2023 Guidelines
Surgical case reports require an emphasis on better completion and quality standards by following specified guidelines. The main changes in the guidelines consist of the following points:
1. Structured Reporting Format
The latest guidelines establish a specific organizational structure for case report writing. The main sections include [2].
A surgical case report requires a title which includes both “case report” terms for easy understanding.
- The reporting structure follows a defined pattern starting with Introduction followed by Case Presentation then Clinical Discussion and concluding with the section on Conclusion.
- The introduction section must explain how the presented case stands out as essential for surgical practice and medical litigation support.
Patient Data: It involves more information about demographics, medical history, and social factors of treatment.
Diagnostic Assessment: Clear documentation of clinical findings, investigations, and decision-making.
Intervention Details: operative steps, difficulties during the intervention, and multidisciplinary efforts.
Follow-Up and Outcomes: Describes short- or long-term patient outcomes using standard assessment metrics.
Discussion: Comparison of the case findings with the existing literature and identifying gaps for future work.
2. Emphasis on Ethical Considerations
Both treatment and publication need patient consent according to the new guidelines [5].
Authors need to include information about ethical approvals together with a discussion of institutional review board (IRB) requirements.
A standardized terminology should be adopted to document both complications and adverse events as it promotes better transparency [6].
Strengthened Follow-up and Outcome Reporting
The guidelines now require authors to include comprehensive details about postoperative patient follow-up, such as the time of visits and the clinician who conducts them.
Authors need to evaluate how well their patients follow their prescribed treatment protocols.
The reporting of complications should use standardized classification systems which include the Clavien-Dindo scale [6].
Multidisciplinary Collaboration and Setting of Care
Complex surgical cases require active involvement from multiple specialties based on the direction provided by the 2023 guidelines [6].
The authors need to present a comprehensive description of the hospital environment that includes district hospitals or specialized trauma centres.
The guidelines emphasize how patient outcomes are impacted by the institution’s expertise and the surgeon’s experience.
How to Use the SCARE 2023 Guidelines?
Authors, researchers, and journal editors should integrate these guidelines into their workflow:
Authors: Authors must use the checklist to achieve complete structured reporting.
Reviewers: Reviewers should employ the guidelines to measure case report quality standards.
Journals: Journals need to demand manuscripts alongside a completed checklist that journals use to verify compliance [5].
Pro Tip: The updated SCARE 2023 checklist is available on the official website (www.scareguideline.com) and can be downloaded in multiple formats for ease of use.
Connect with us to explore how we can support you in maintaining academic integrity and enhancing the visibility of your research across the world!
We offer the expertise, knowledge, and comprehensive support your Clinical research and publication needs.
Impact of the Updated Guidelines
The Impact of the SCARE Guidelines
The SCARE guidelines show effectiveness by improving the quality of case reports. The updated guidelines improve the effectiveness of case reports for medical litigation support. Journals that follow standardized reporting requirements create reports of superior quality, thoroughness, and consistency [6].
By following the SCARE 2023 guidelines, authors will:
- Improve clarity and accuracy in case reporting.
- Enhance the educational value of published reports.
- Facilitate the reproducibility of surgical techniques and patient outcomes [6].
Conclusion
The SCARE 2023 guidelines establish an ethical framework that provides standardized guidelines for surgical case report reporting. The guidelines enhance documentation practices which leads to better credibility and increased impact of case reports in medical literature. This literature evidence can support in the medico-legal services and medical litigation support.
Key Takeaways:
Improved framework for documenting case details.
Stronger focus on ethics, patient consent, and follow-up.
Assist experts for medico-legal advisory services.
Promotion of multidisciplinary collaboration.
Checklist-based compliance for authors and reviewers.
Next Step? Download the SCARE 2023 checklist and integrate it into your surgical reporting process today!