Harvesting clinical data
Evidence-based consulting is the new "normal" in the healthcare industry. Better consulting is possible when you get your evidence right; therefore, tap in to the clinical data of patients. Clinical Data Management (CDM)—a pivotal area of clinical research; let Scientific Writing & Publishing lead the way.

Specific Technologies
Commitment to a process driven yet customized approach. We run clinical trials on Electronic Data Capture (EDC), Clinical Trial Management System (CTMS), eTMF platforms and import data from various devices.
Regulatory Compliance
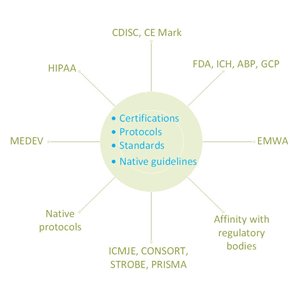
Absolute compliance with international standards to ensure consistency in clinical data. Use of data management best practices and tools.
Industry Standards
The data definitions are prepared and provided in line with Clinical Data Interchange Standards Consortium (CDISC) Case Report Tabulation and Data Definition (CRT-DD) specifications and standards. All our experts are certified.
EVIDENCE-BASED CONSULTING: SERVICES
1.Discuss procedures and specifications and prepare operating procedures
2.Prepare data base (DB) definition, data input standards, and other specifications
3.Clinical Trial Management System (CDMS) setup.
4.Data validation.
EVIDENCE-BASED CONSULTING: SERVICES
1. Case Report Form (CRF) copies and PDFs.
2. Data input, comparing and coding.
3. Logical and manual checks.
4. Prepare material for investigation and CRF lock (primary).
EVIDENCE-BASED CONSULTING: SERVICES
1. Matching Adverse Events (AE
2. Prepare case materials for case review.
3. Generate SAS data sets.
EVIDENCE-BASED CONSULTING: SERVICES
1. Generate Data Manatement (DM) log sheets.
2. Self inspection on completion--anually or on completion.
3. Record retention or archive management.
PUBLICATION QUALITY ASSURANCE GUARANTEED


Niche Experts
More than 1000 subject-matter experts. Let our experts call the shots.

Certified Writers
More than 15 years of editorial experience. Leave the writing to us.

Multiple Domains
Served more than 20,000 academic institutions .
Hasten you projects through our experts.
Prolific writers across plethora of areas who know your subject and industry.
Seamless support.
We are with you the whole nine yards of the publishing process.